Nutr Res Pract.
2024 Feb;18(1):62-77. 10.4162/nrp.2024.18.1.62.
Combination of oxaliplatin and β-carotene suppresses colorectal cancer by regulating cell cycle, apoptosis, and cancer stemness in vitro
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 03760, Korea
- 2Department of Surgery, Seoul National University-Seoul Metropolitan Government (SNU-SMG) Boramae Medical Center, Seoul 07061, Korea
- 3Graduate Program in System Health Science and Engineering, Ewha Womans University, Seoul 03760, Korea
- KMID: 2552280
- DOI: http://doi.org/10.4162/nrp.2024.18.1.62
Abstract
- BACKGROUND/OBJECTIVES
Colorectal cancer (CRC) is the third most common cancer worldwide with a high recurrence rate. Oxaliplatin (OXA) resistance is one of the major reasons hindering CRC therapy. β-Carotene (BC) is a provitamin A and is known to have antioxidant and anticancer effects. However, the combined effect of OXA and BC has not been investigated. Therefore, this study investigated the anticancer effects and mechanism of the combination of OXA and BC on CRC.
MATERIALS/METHODS
In the present study, the effects of the combination of OXA and BC on cell viability, cell cycle arrest, and cancer stemness were investigated using HCT116, HT29, OXA-resistant cells, and human CRC organoids.
RESULTS
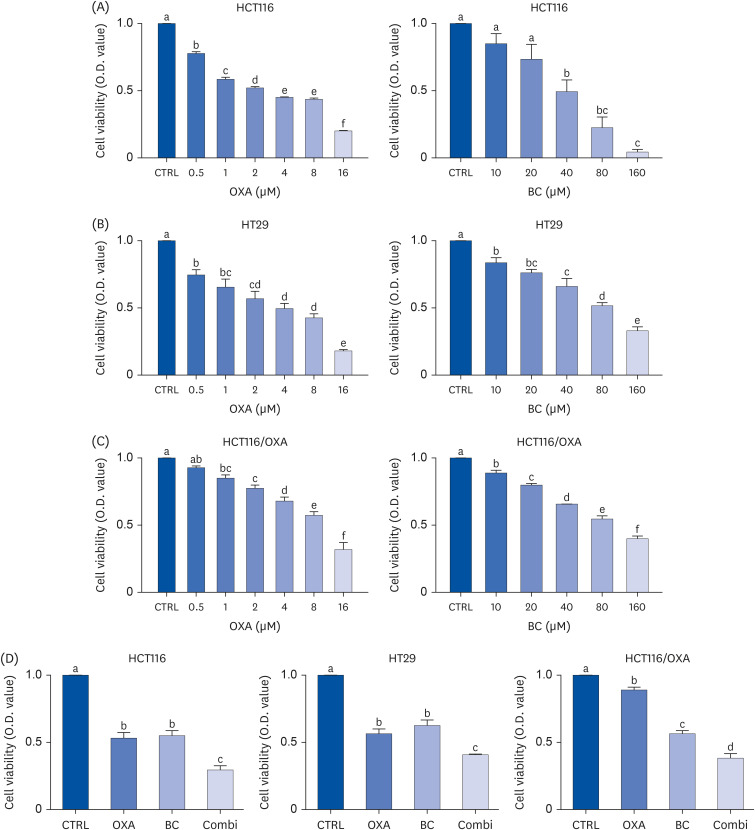
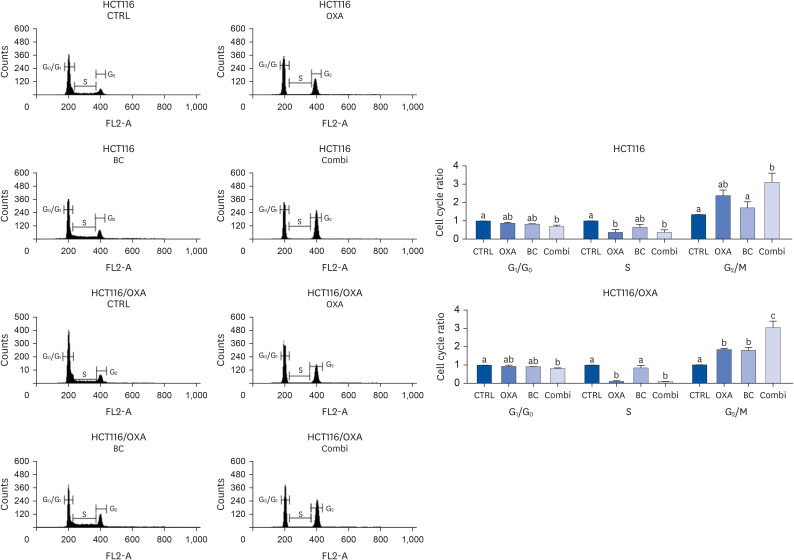
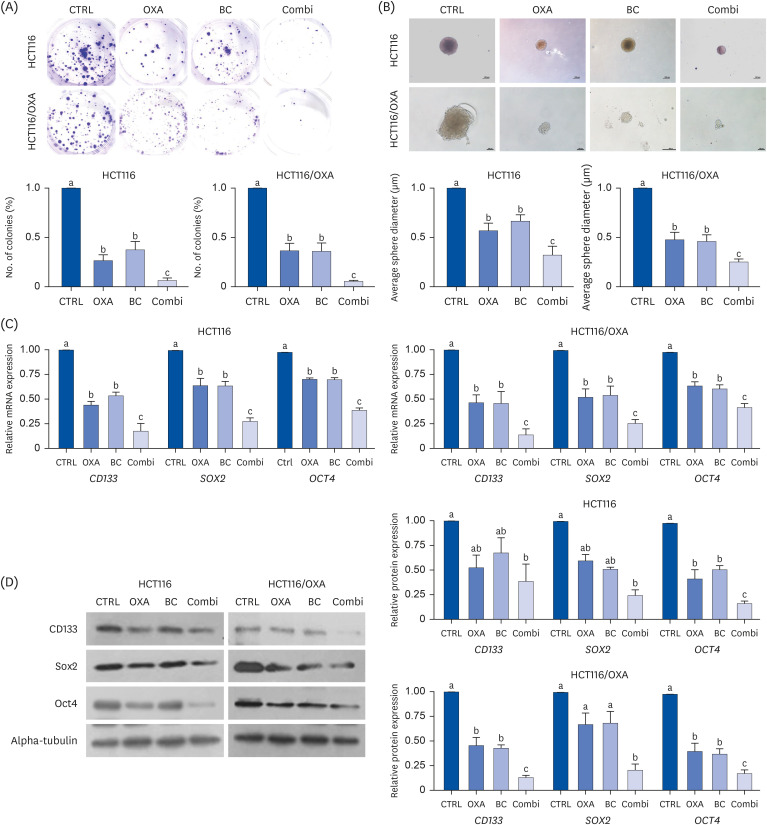
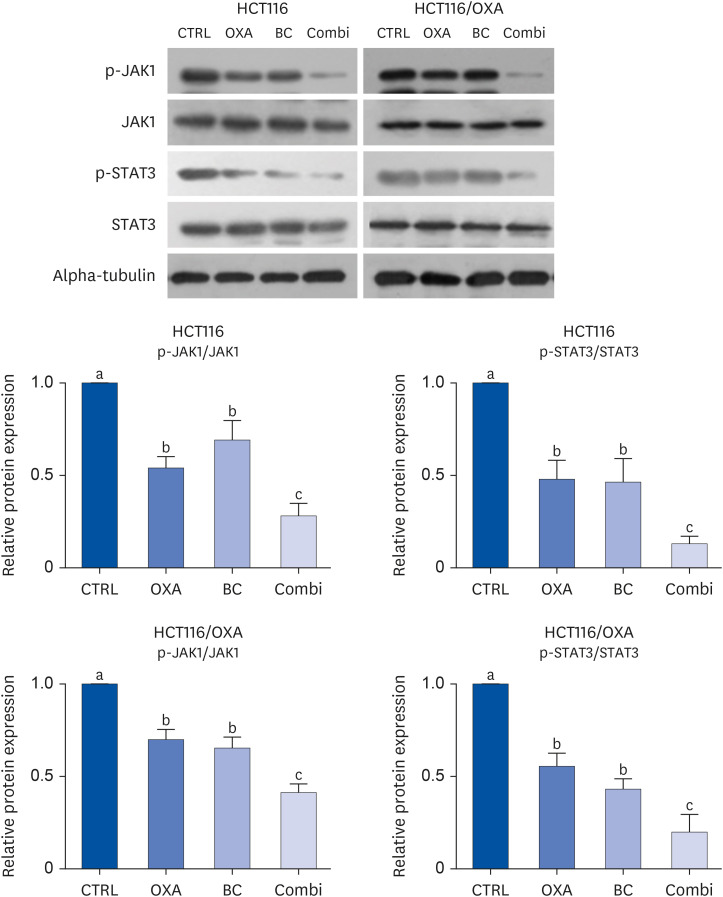
The combination of OXA and BC enhanced apoptosis, G 2,/sub> /M phase cell cycle arrest, and inhibited cancer cell survival in human CRC resistant cells and CRC organoids without toxicity in normal organoids. Cancer stem cell marker expression and self-replicating capacity were suppressed by combined treatment with OXA and BC. Moreover, this combined treatment upregulated apoptosis and the stem cell-related JAK/STAT signaling pathway.
CONCLUSIONS
Our results suggest a novel potential role of BC in reducing resistance to OXA, thereby enhances the anticancer effects of OXA. This enhancement is achieved through the regulation of cell cycle, apoptosis, and stemness in CRC.
Keyword
Figure
Reference
-
1. Jung KW, Won YJ, Kang MJ, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2022. Cancer Res Treat. 2022; 54:345–351. PMID: 35313101.2. Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016; 8:57–84. PMID: 26753006.3. Nagourney RA, Evans S, Tran PH, Nagourney AJ, Sugarbaker PH. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur J Surg Oncol. 2021; 47:738–742. PMID: 33004272.4. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003; 22:7265–7279. PMID: 14576837.5. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011; 17:313–319. PMID: 21386835.6. Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (Dordr). 2020; 43:123–136. PMID: 31713003.7. Zhang HL, Wang P, Lu MZ, Zhang SD, Zheng L. c-Myc maintains the self-renewal and chemoresistance properties of colon cancer stem cells. Oncol Lett. 2019; 17:4487–4493. PMID: 30944638.8. Tang S, Yuan X, Song J, Chen Y, Tan X, Li Q. Association analyses of the JAK/STAT signaling pathway with the progression and prognosis of colon cancer. Oncol Lett. 2019; 17:159–164. PMID: 30655751.9. Xiong W, Dong J, Kong S. Dentatin exerts anticancer effects on human colon cancer cell lines via cell cycle arrest, autophagy, inhibition of cell migration and JAK/STAT signalling pathway. J BUON. 2019; 24:1488–1493. PMID: 31646796.10. Houssein M, Abi Saab W, Khalil M, Khalife H, Fatfat M. Cell death by gallotannin is associated with inhibition of the JAK/STAT pathway in human colon cancer cells. Curr Ther Res Clin Exp. 2020; 92:100589. PMID: 32714471.11. Taylor PR, Li B, Dawsey SM, Li JY, Yang CS, Guo W, Blot WJ. Prevention of esophageal cancer: the nutrition intervention trials in Linxian, China. Linxian Nutrition Intervention Trials Study Group. Cancer Res. 1994; 54:2029s–2031s. PMID: 8137333.12. Palozza P, Serini S, Maggiano N, Angelini M, Boninsegna A, Di Nicuolo F, Ranelletti FO, Calviello G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis. 2002; 23:11–18. PMID: 11756218.13. Cui Y, Lu Z, Bai L, Shi Z, Zhao WE, Zhao B. Beta-carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer. 2007; 43:2590–2601. PMID: 17911009.14. Lim JY, Kim YS, Kim KM, Min SJ, Kim Y. B-carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness. Biochem Biophys Res Commun. 2014; 450:1475–1480. PMID: 25019987.15. Zhang X, Zhao WE, Hu L, Zhao L, Huang J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch Biochem Biophys. 2011; 512:96–106. PMID: 21620794.16. Ngoc NB, Lv P, Zhao WE. Suppressive effects of lycopene and β-carotene on the viability of the human esophageal squamous carcinoma cell line EC109. Oncol Lett. 2018; 15:6727–6732. PMID: 29731858.17. Lee HA, Park S, Kim Y. Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells. Oncol Rep. 2013; 30:1869–1877. PMID: 23900747.18. Lee KE, Kwon M, Kim YS, Kim Y, Chung MG, Heo SC, Kim Y. β-carotene regulates cancer stemness in colon cancer in vivo and in vitro. Nutr Res Pract. 2022; 16:161–172. PMID: 35392530.19. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006; 1:2315–2319. PMID: 17406473.20. Kim E, Shin JH, Seok PR, Kim MS, Yoo SH, Kim Y. Phyllodulcin, a natural functional sweetener, improves diabetic metabolic changes by regulating hepatic lipogenesis, inflammation, oxidative stress, fibrosis, and gluconeogenesis in db/db mice. J Funct Foods. 2018; 42:1–11.21. Cristobal A, van den Toorn HWP, van de Wetering M, Clevers H, Heck AJR, Mohammed S. Personalized proteome profiles of healthy and tumor human colon organoids reveal both individual diversity and basic features of colorectal cancer. Cell Reports. 2017; 18:263–274. PMID: 28052255.22. Michels BE, Mosa MH, Grebbin BM, Yepes D, Darvishi T, Hausmann J, Urlaub H, Zeuzem S, Kvasnicka HM, Oellerich T, et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. J Exp Med. 2019; 216:704–720. PMID: 30792186.23. Howells LM, Mitra A, Manson MM. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int J Cancer. 2007; 121:175–183. PMID: 17330230.24. Palozza P, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Calviello G. Beta-carotene downregulates the steady-state and heregulin-alpha-induced COX-2 pathways in colon cancer cells. J Nutr. 2005; 135:129–136. PMID: 15623844.25. Upadhyaya KR, Radha KS, Madhyastha HK. Cell cycle regulation and induction of apoptosis by beta-carotene in U937 and HL-60 leukemia cells. J Biochem Mol Biol. 2007; 40:1009–1015. PMID: 18047798.26. Sowmya Shree G, Yogendra Prasad K, Arpitha HS, Deepika UR, Nawneet Kumar K, Mondal P, Ganesan P. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol Cell Biochem. 2017; 436:1–12. PMID: 28550445.27. Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, Hendlisz A, Van Laethem JL. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer. 2006; 94:481–485. PMID: 16434988.28. Kim D, Kim Y, Kim Y. Effects of β-carotene on expression of selected microRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J Cancer Prev. 2019; 24:224–232. PMID: 31950022.29. Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008; 10:287–297. PMID: 18320073.30. Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006; 1091:151–169. PMID: 17341611.31. Yu L, Li J, Xiao M. Picrocrocin exhibits growth inhibitory effects against SKMEL-2 human malignant melanoma cells by targeting JAK/STAT5 signaling pathway, cell cycle arrest and mitochondrial mediated apoptosis. J BUON. 2018; 23:1163–1168. PMID: 30358226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of β-carotene on Cell Growth Inhibition of KB Human Oral Cancer Cells
- Hypersensitivity Reactions to Oxaliplatin
- Long Non-Coding RNA LINC00525 Promotes the Stemness and Chemoresistance of Colorectal Cancer by Targeting miR-507/ELK3 Axis
- Ribosomal Protein L9 Maintains Stemness of Colorectal Cancer via an ID-1 Dependent Mechanism
- Change in Expression of Survivin Caused by Using Oxaliplatin in HCT116 Colon Cancer Cells