Ann Lab Med.
2023 Nov;43(6):605-613. 10.3343/alm.2023.43.6.605.
Cost-Effectiveness Analysis of Three Diagnostic Strategies for the Detection of EGFR Mutation in Advanced Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Laboratory Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 2Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, National Cancer Center, Goyang, Korea
- 5Departments of Laboratory Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- KMID: 2552038
- DOI: http://doi.org/10.3343/alm.2023.43.6.605
Abstract
- Background
In non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR) mutation testing of tumor tissue should be conducted at diagnosis. Alternatively, circulating tumor DNA can be used to detect EGFR mutation. We compared the cost and clinical effect of three strategies according to the application of the EGFR test.
Methods
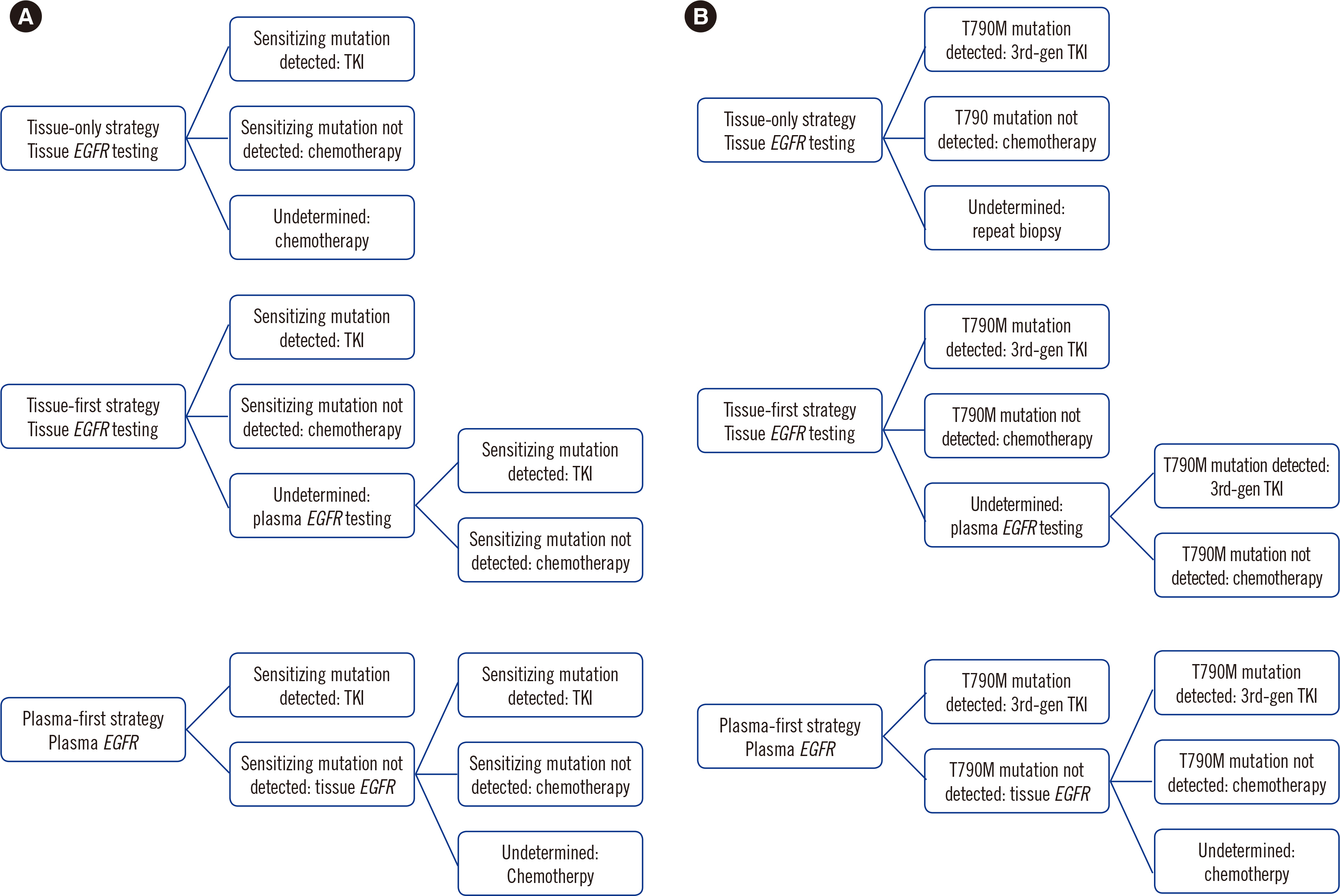
Decision models were developed to compare the cost-effectiveness of tissue-only, tissue-first, and plasma-first diagnostic strategies as first- and second-line treatments for NSCLC from the perspective of the Korean national healthcare payer. Progression-free survival (PFS), overall survival (OS), and direct medical costs were assessed. A one-way sensitivity analysis was performed.
Results
The plasma-first strategy correctly identified numerous patients in the first- and second-line treatments. This strategy also decreased the cost of biopsy procedures and complications. Compared with that when using the other two strategies, the plasma-first strategy increased PFS by 0.5 months. The plasma-first strategy increased OS by 0.9 and 1 month compared with that when using the tissue-only and tissue-first strategies, respectively. The plasma-first strategy was the least expensive first-line treatment but the most expensive second-line treatment. First-generation tyrosine kinase inhibitor and the detection rate of the T790M mutation in tissues were the most cost-influential factors.
Conclusions
The plasma-first strategy improved PFS and OS, allowing for a more accurate identification of candidates for targeted therapy for NSCLC and decreased biopsy- and complication-related costs.
Keyword
Figure
Cited by 1 articles
-
Next-Generation Sequencing-Based Molecular Profiling Using Cell-Free DNA: A Valuable Tool for the Diagnostic and Prognostic Evaluation of Patients With Gastric Cancer
Mi-Ae Jang
Ann Lab Med. 2024;44(2):119-121. doi: 10.3343/alm.2023.0391.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. 2015; Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–86. DOI: 10.1002/ijc.29210. PMID: 25220842.
Article2. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2017. Ministry of Health and Welfare;2020.3. Lim C, Sung M, Shepherd FA, Nouriany N, Sawczak M, Paul T, et al. 2016; Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials? J Thorac Oncol. 11:79–84. DOI: 10.1016/j.jtho.2015.09.006. PMID: 26762742.
Article4. Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. 2005; Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 23:5900–9. DOI: 10.1200/JCO.2005.02.857. PMID: 16043828.
Article5. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. 2013; Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 19:2240–7. DOI: 10.1158/1078-0432.CCR-12-2246. PMID: 23470965. PMCID: PMC3630270.6. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. 2014; AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4:1046–61. DOI: 10.1158/2159-8290.CD-14-0337. PMID: 24893891. PMCID: PMC4315625.
Article7. Goldman JW, Noor ZS, Remon J, Besse B, Rosenfeld N. 2018; Are liquid biopsies a surrogate for tissue EGFR testing? Ann Oncol. 29(Suppl 1):i38–i46. DOI: 10.1093/annonc/mdx706. PMID: 29462257.8. Goto K, Ichinose Y, Ohe Y, Yamamoto N, Negoro S, Nishio K, et al. 2012; Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 7:115–21. DOI: 10.1097/JTO.0b013e3182307f98. PMID: 21900837.
Article9. Diaz LA Jr. 2014; and Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 32:579–86. DOI: 10.1200/JCO.2012.45.2011. PMID: 24449238. PMCID: PMC4820760.10. Karlovich C, Goldman JW, Sun JM, Mann E, Sequist LV, Konopa K, et al. 2016; Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase I study of rociletinib (CO-1686). Clin Cancer Res. 22:2386–95. DOI: 10.1158/1078-0432.CCR-15-1260. PMID: 26747242. PMCID: PMC6886231.11. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. 2009; Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361:947–57. DOI: 10.1056/NEJMoa0810699. PMID: 19692680.
Article12. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. 2011; Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 29:2866–74. DOI: 10.1200/JCO.2010.33.4235. PMID: 21670455.
Article13. Wu Y-L, Mok TSK, Han J-Y, Ahn M-J, Delmonte A, Ramalingam SS, et al. 2019; Overall survival (OS) from the AURA3 phase III study: osimertinib vs platinum-pemetrexed (plt-pem) in patients (pts) with EGFR T790M advanced non-small cell lung cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI). Ann Oncol. 30:IX158. DOI: 10.1093/annonc/mdz437.001.14. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. 2017; Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 376:629–40. DOI: 10.1056/NEJMoa1612674. PMID: 27959700. PMCID: PMC6762027.
Article15. Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. 2016; Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 34:3375–82. DOI: 10.1200/JCO.2016.66.7162. PMID: 27354477. PMCID: PMC5035123.
Article16. Sands J, Li Q, Hornberger J. 2017; Urine circulating-tumor DNA (ctDNA) detection of acquired EGFR T790M mutation in non-small-cell lung cancer: an outcomes and total cost-of-care analysis. Lung Cancer. 110:19–25. DOI: 10.1016/j.lungcan.2017.05.014. PMID: 28676213.
Article17. Jenkins S, Yang JCH, Ramalingam SS, Yu K, Patel S, Weston S, et al. 2017; Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol. 12:1061–70. DOI: 10.1016/j.jtho.2017.04.003. PMID: 28428148.18. Thress KS, Brant R, Carr TH, Dearden S, Jenkins S, Brown H, et al. 2015; EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 90:509–15. DOI: 10.1016/j.lungcan.2015.10.004. PMID: 26494259.
Article19. Goldman JW, Karlovich C, Sequist LV, Melnikova V, Franovic A, Gadgeel SM, et al. 2018; EGFR genotyping of matched urine, plasma, and tumor tissue in patients with non-small-cell lung cancer treated with rociletinib, an EGFR tyrosine kinase inhibitor. JCO Precis Oncol. 2:1–13. DOI: 10.1200/PO.17.00116. PMID: 35135111.20. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M. 2017; Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 27:138–48. DOI: 10.1007/s00330-016-4357-8. PMID: 27108299. PMCID: PMC5127875.
Article21. Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT. 2004; Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 15:479–83. DOI: 10.1097/01.RVI.0000124951.24134.50. PMID: 15126658.
Article22. Wiener RS, Schwartz LM, Woloshin S, Welch HG. 2011; Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 155:137–44. DOI: 10.7326/0003-4819-155-3-201108020-00003. PMID: 21810706. PMCID: PMC3150964.
Article23. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer, Version 5. 2021.24. Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, et al. 2021; Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 16:1647–62. DOI: 10.1016/j.jtho.2021.06.017. PMID: 34246791.
Article25. Gancitano G, Ravasio R, Dionisi M, Cortinovis D. Cost-consequence analysis of three different diagnostic strategies in the first- and second-line treatment of locally advanced or metastatic non-small-cell lung cancer. Farmeconomia, Health Economics and Therapeutic Pathways. 2018; 19:DOI: 10.7175/fe.v19i1.1354.
Article26. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. 2010; Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 362:2380–8. DOI: 10.1056/NEJMoa0909530. PMID: 20573926.
Article27. Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. 2013; Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 24:54–9. DOI: 10.1093/annonc/mds214. PMID: 22967997.28. Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, et al. 2016; Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2:1014–22. DOI: 10.1001/jamaoncol.2016.0173. PMID: 27055085. PMCID: PMC4982795.
Article29. Sorber L, Zwaenepoel K, Deschoolmeester V, Van Schil PE, Van Meerbeeck J, Lardon F, et al. 2017; Circulating cell-free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer. 107:100–7. DOI: 10.1016/j.lungcan.2016.04.026. PMID: 27180141.
Article30. Papadimitrakopoulou VA, Han JY, Ahn MJ, Ramalingam SS, Delmonte A, Hsia TC, et al. 2020; Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer. 126:373–80. DOI: 10.1002/cncr.32503. PMID: 31769875.
Article31. Kim B, Kim Y, Shin S, Lee ST, Cho JY, Lee KA. 2022; Application of CRISPR/Cas9-based mutant enrichment technique to improve the clinical sensitivity of plasma EGFR testing in patients with non-small cell lung cancer. Cancer Cell Int. 22:82. DOI: 10.1186/s12935-022-02504-2. PMID: 35168603. PMCID: PMC8845274.32. Kim Y, Shin S, Lee KA. 2021; Exosome-based detection of EGFR T790M in plasma and pleural fluid of prospectively enrolled non-small cell lung cancer patients after first-line tyrosine kinase inhibitor therapy. Cancer Cell Int. 21:50. DOI: 10.1186/s12935-021-01761-x. PMID: 33435996. PMCID: PMC7802208.33. Shin S, Woo HI, Kim JW, Kim Y, Lee KA. 2022; Clinical practice guidelines for pre-analytical procedures of plasma epidermal growth factor receptor variant testing. Ann Lab Med. 42:141–9. DOI: 10.3343/alm.2022.42.2.141. PMID: 34635607. PMCID: PMC8548242.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Should We Perform Repeated Re-biopsy for the Detection of T790M Mutation?
- Detection of EGFR and KRAS Mutation by Pyrosequencing Analysis in Cytologic Samples of Non-Small Cell Lung Cancer
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- A Case of Patient with Lung Adenocarcinoma with Double Rare EGFR Mutation of G719C and L861Q
- EGFR C797S as a Resistance Mechanism of Lazertinib in Non-small Cell Lung Cancer with EGFR T790M Mutation