Ann Lab Med.
2023 Jul;43(4):364-374. 10.3343/alm.2023.43.4.364.
Impact of Low-Level Donor-Specific Anti-HLA Antibody on Posttransplant Clinical Outcomes in Kidney Transplant Recipients
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Nephrology, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 3Transplant Research Center, Convergent Research Consortium for Immunologic Disease, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Division of Nephrology, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea
- 5Department of Laboratory Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2551954
- DOI: http://doi.org/10.3343/alm.2023.43.4.364
Abstract
- Background
The clinical significance of low-level donor-specific anti-HLA antibody (low-DSA) remains controversial. We investigated the impact of low-DSA on posttransplant clinical outcomes in kidney transplant (KT) recipients.
Methods
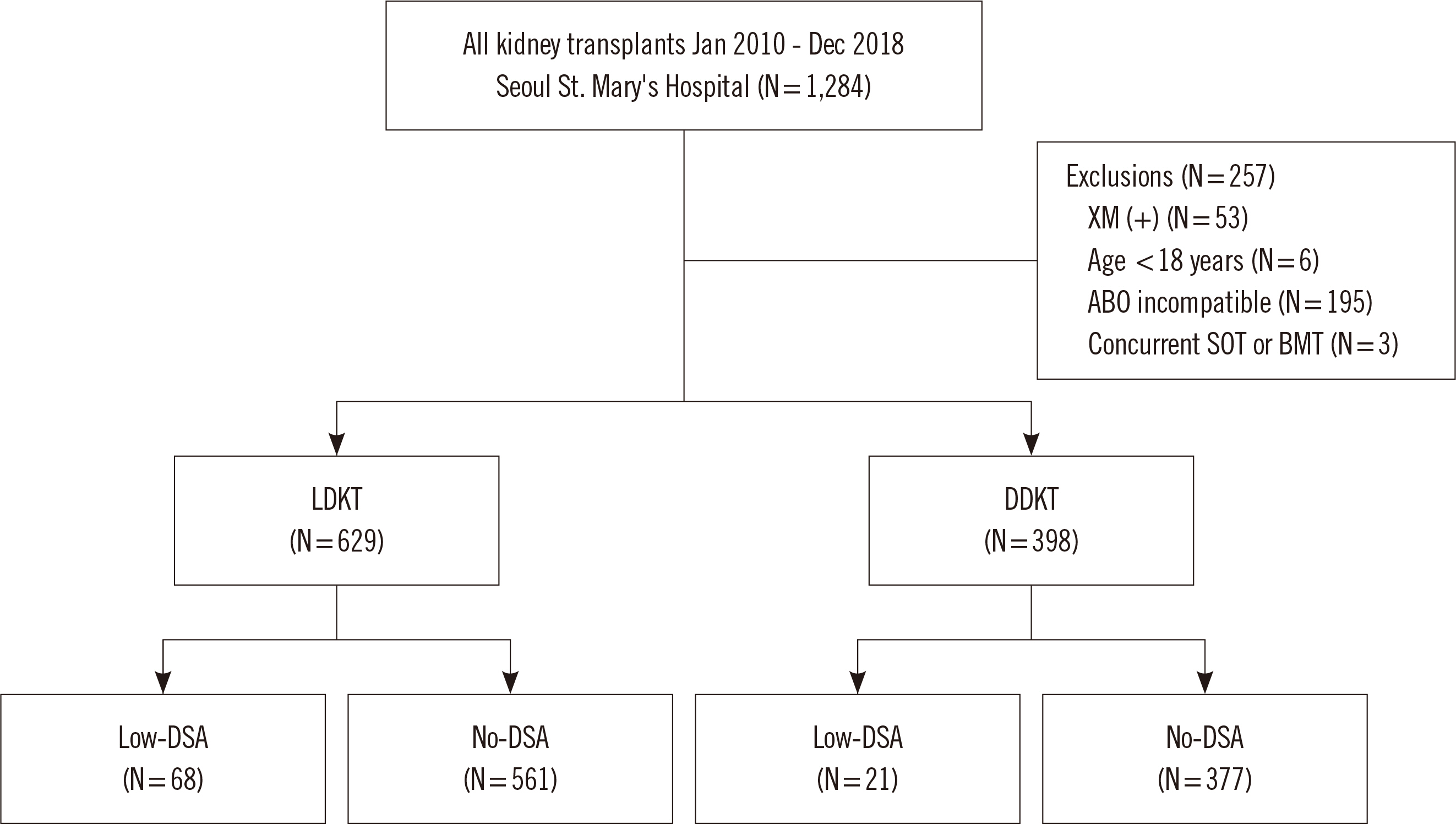
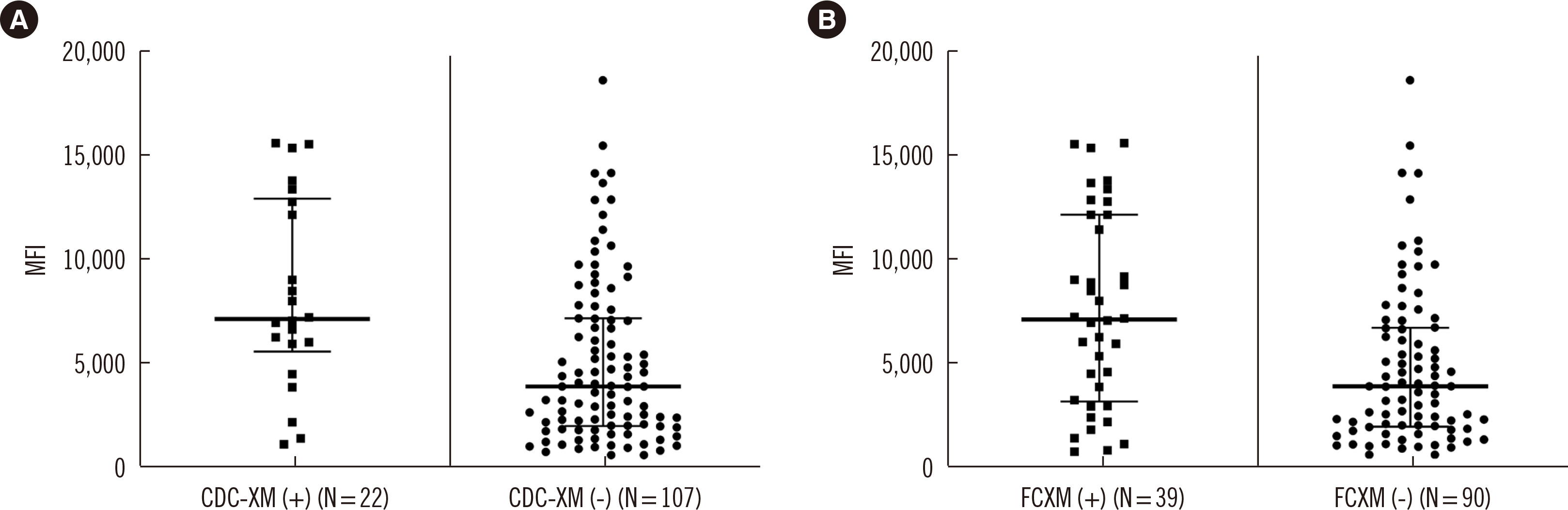
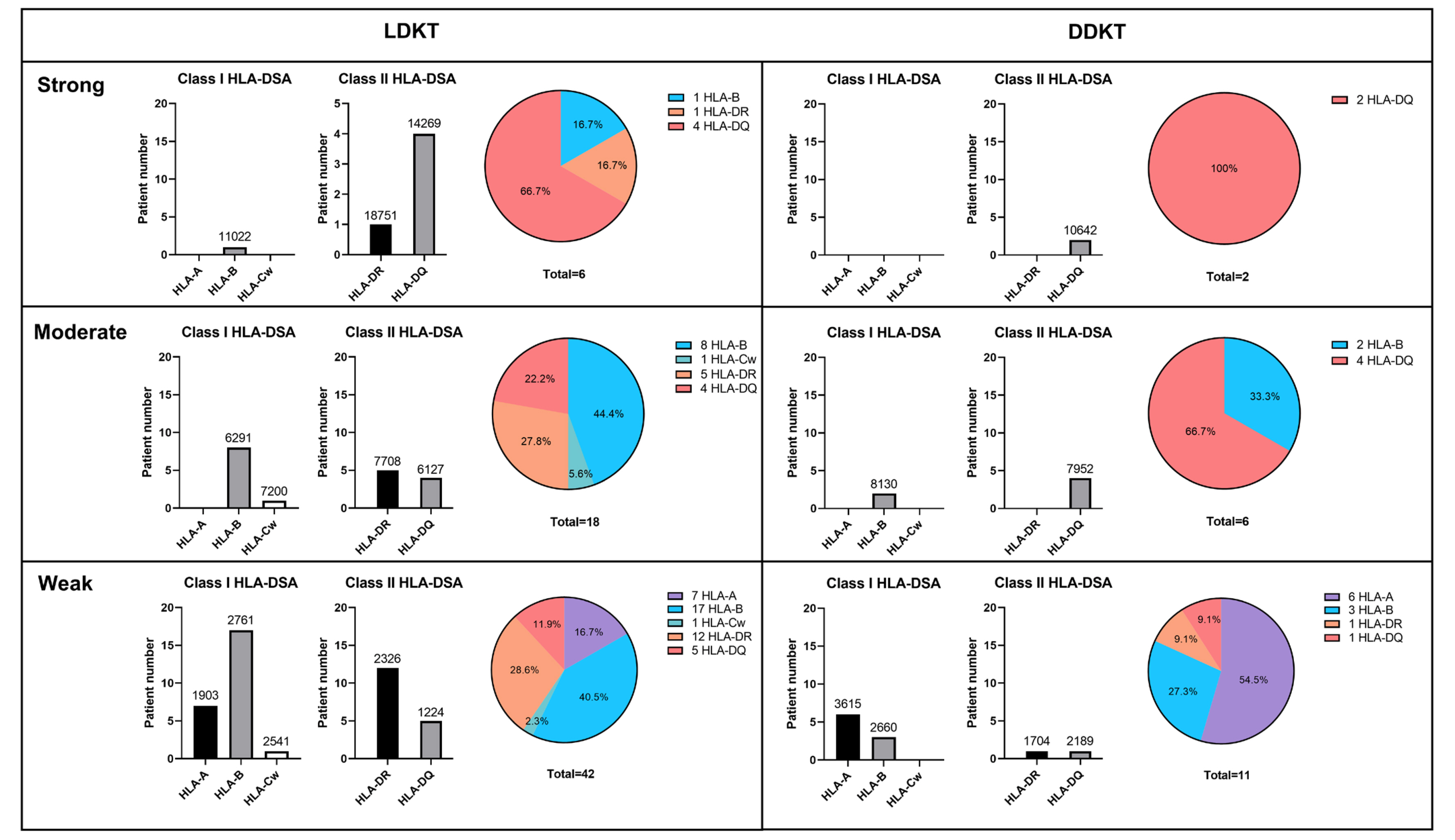
We retrospectively reviewed 1,027 KT recipients, namely, 629 living donor KT (LDKT) recipients and 398 deceased donor KT (DDKT) recipients, in Seoul St. Mary’s Hospital (Seoul, Korea) between 2010 and 2018. Low-DSA was defined as a positive anti-HLA-DSA result in the Luminex single antigen assay (LABScreen single antigen HLA class I - combi and class II - group 1 kits; One Lambda, Canoga Park, CA, USA) but a negative result in a crossmatch test. We compared the incidence of biopsy-proven allograft rejection (BPAR), changes in allograft function, allograft survival, patient survival, and posttransplant infections between subgroups according to pretransplant low-DSA.
Results
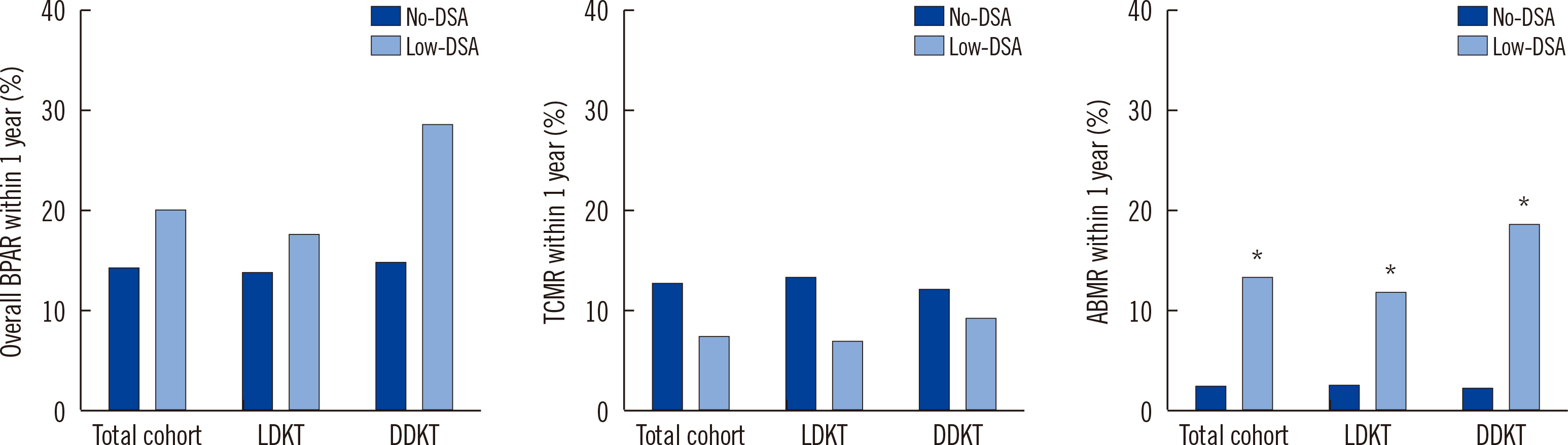
The incidence of overall BPAR and T cell-mediated rejection did not differ between the subgroups. However, antibody-mediated rejection (ABMR) developed more frequently in patients with low-DSA than in those without low-DSA in the total cohort and the LDKT and DDKT subgroups. In multivariate analysis, low-DSA was identified as a risk factor for ABMR development. Its impact was more pronounced in DDKT (odds ratio [OR]: 9.60, 95% confidence interval [CI]: 1.79–51.56) than in LDKT (OR: 3.76, 95% CI: 0.99–14.26) recipients. There were no significant differences in other outcomes according to pretransplant low-DSA.
Conclusions
Pretransplant low-DSA has a significant impact on the development of ABMR, and more so in DDKT recipients than in LDKT recipients, but not on long-term outcomes.
Keyword
Figure
Cited by 1 articles
-
Impact of Low-level Donor-specific Antibody Determined With a Positive Luminex and Negative Flow Cytometric Crossmatch on Kidney Transplantation Outcomes
Minjeong Nam, Eun Young Song
Ann Lab Med. 2023;43(4):325-327. doi: 10.3343/alm.2023.43.4.325.
Reference
-
1. Patel R, Terasaki PI. 1969; Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 280:735–9. DOI: 10.1056/NEJM196904032801401. PMID: 4886455.
Article2. Stiller CR, Sinclair NR, Abrahams S, Ulan RA, Fung M, Wallace AC. 1975; Lymphocyte-dependent antibody and renal graft rejection. Lancet. 1:953–4. DOI: 10.1016/S0140-6736(75)92010-3. PMID: 48125.
Article3. Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. 2014; Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 14:1573–80. DOI: 10.1111/ajt.12786. PMID: 24913913.
Article4. Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. 2013; Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 95:19–47. DOI: 10.1097/TP.0b013e31827a19cc. PMID: 23238534.
Article5. Johnson AH, Rossen RD, Butler WT. 1972; Detection of alloantibodies using a sensitive antiglobulin microcytotoxicity test: identification of low levels of pre-formed antibodies in accelerated allograft rejection. Tissue Antigens. 2:215–26. DOI: 10.1111/j.1399-0039.1972.tb00138.x. PMID: 4586478.
Article6. Couzi L, Araujo C, Guidicelli G, Bachelet T, Moreau K, Morel D, et al. 2011; Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 91:527–35. DOI: 10.1097/TP.0b013e31820794bb. PMID: 21192319.
Article7. Batal I, Zeevi A, Lunz JG 3rd, Aggarwal N, Shapiro R, Randhawa P, et al. 2010; Antihuman leukocyte antigen-specific antibody strength determined by complement-dependent or solid-phase assays can predict positive donor-specific crossmatches. Arch Pathol Lab Med. 134:1534–40. DOI: 10.5858/2009-0581-OA.1. PMID: 20923311.
Article8. Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. 2010; Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 21:1398–406. DOI: 10.1681/ASN.2009101065. PMID: 20634297. PMCID: PMC2938596.
Article9. Zecher D, Bach C, Staudner C, Böger CA, Bergler T, Banas B, et al. 2017; Characteristics of donor-specific anti-HLA antibodies and outcome in renal transplant patients treated with a standardized induction regimen. Nephrol Dial Transplant. 32:730–7. DOI: 10.1093/ndt/gfw445. PMID: 28339671.
Article10. Mujtaba MA, Goggins W, Lobashevsky A, Sharfuddin AA, Yaqub MS, Mishler DP, et al. 2011; The strength of donor-specific antibody is a more reliable predictor of antibody-mediated rejection than flow cytometry crossmatch analysis in desensitized kidney recipients. Clin Transplant. 25:E96–102. DOI: 10.1111/j.1399-0012.2010.01341.x. PMID: 20977497.
Article11. Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. 2011; Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 365:318–26. DOI: 10.1056/NEJMoa1012376. PMID: 21793744.
Article12. Sethi S, Choi J, Toyoda M, Vo A, Peng A, Jordan SC. 2017; Desensitization: overcoming the immunologic barriers to transplantation. J Immunol Res. 2017:6804678. DOI: 10.1155/2017/6804678. PMID: 28127571. PMCID: PMC5239985.
Article13. Park Y, Ko EJ, Chung BH, Yang CW. 2021; Kidney transplantation in highly sensitized recipients. Kidney Res Clin Pract. 40:355–70. DOI: 10.23876/j.krcp.21.012. PMID: 34233438. PMCID: PMC8476304.
Article14. Salvadé I, Aubert V, Venetz JP, Golshayan D, Saouli AC, Matter M, et al. 2016; Clinically-relevant threshold of preformed donor-specific anti-HLA antibodies in kidney transplantation. Hum Immunol. 77:483–9. DOI: 10.1016/j.humimm.2016.04.010. PMID: 27085791.
Article15. Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. 2012; Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 23:2061–71. DOI: 10.1681/ASN.2012070664. PMID: 23160511. PMCID: PMC3507372.
Article16. Buttigieg J, Ali H, Sharma A, Halawa A. 2019; Positive Luminex and negative flow cytometry in kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 34:1950–60. DOI: 10.1093/ndt/gfy349. PMID: 30508114.
Article17. Malheiro J, Tafulo S, Dias L, Martins LS, Fonseca I, Beirão I, et al. 2015; Analysis of preformed donor-specific anti-HLA antibodies characteristics for prediction of antibody-mediated rejection in kidney transplantation. Transpl Immunol. 32:66–71. DOI: 10.1016/j.trim.2015.01.002. PMID: 25661873.
Article18. Chung BH, Choi BS, Oh EJ, Park CW, Kim JI, Moon IS, et al. 2014; Clinical impact of the baseline donor-specific anti-human leukocyte antigen antibody measured by Luminex single antigen assay in living donor kidney transplant recipients after desensitization therapy. Transpl Int. 27:49–59. DOI: 10.1111/tri.12199. PMID: 24118413.
Article19. Kim Y, Yang CW, Moon IS, Kim M, Lim J, Park YJ, et al. 2010; Donor-specific HLA class I and CREG antibodies in complement-dependent cytotoxicity-negative renal transplants. Ann Clin Lab Sci. 40:330–5.20. Jang JY, Kim YJ, Kim Y, Park YJ, Han K, Oh EJ. 2012; Application of calculated panel reactive antibody using HLA frequencies in Koreans. Ann Lab Med. 32:66–72. DOI: 10.3343/alm.2012.32.1.66. PMID: 22259781. PMCID: PMC3255493.
Article21. Hwang SD, Chung BH, Oh EJ, Choi BS, Park CW, Kim YS, et al. 2015; Effect of pretransplant rituximab use on posttransplant clinical outcomes in patients with high panel reactive antibody scores. Nephron. 130:239–44. DOI: 10.1159/000435924. PMID: 26182858.
Article22. Loupy A, Mengel M, Haas M. 2022; Thirty years of the International Banff Classification for Allograft Pathology: the past, present, and future of kidney transplant diagnostics. Kidney Int. 101:678–91. DOI: 10.1016/j.kint.2021.11.028. PMID: 34922989.
Article23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009; A new equation to estimate glomerular filtration rate. Ann Intern Med. 150:604–12. DOI: 10.7326/0003-4819-150-9-200905050-00006. PMID: 19414839. PMCID: PMC2763564.
Article24. Razonable RR, Humar A. 2019; Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 33:e13512. DOI: 10.1111/ctr.13512. PMCID: PMC6635088.
Article25. Hirsch HH, Randhawa PS. AST Infectious Diseases Community of Practice. 2019; BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 33:e13528. DOI: 10.1111/ctr.13528.
Article26. Higgins R, Lowe D, Hathaway M, Williams C, Lam FT, Kashi H, et al. 2011; Human leukocyte antigen antibody-incompatible renal transplantation: excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 92:900–6. DOI: 10.1097/TP.0b013e31822dc38d. PMID: 21968524.
Article27. Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al. 2018; Differential effects of donor-specific HLA antibodies in living versus deceased donor transplant. Am J Transplant. 18:2274–84. DOI: 10.1111/ajt.14709. PMID: 29464832. PMCID: PMC6175247.
Article28. Ziemann M, Altermann W, Angert K, Arns W, Bachmann A, Bakchoul T, et al. 2019; Preformed donor-specific HLA antibodies in living and deceased donor transplantation: a multicenter study. Clin J Am Soc Nephrol. 14:1056–66. DOI: 10.2215/CJN.13401118. PMID: 31213508. PMCID: PMC6625630.29. Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, et al. 2021; OPTN/SRTR 2019 annual data report: kidney. Am J Transplant. 21(Suppl 2):21–137. DOI: 10.1111/ajt.16502. PMID: 33595191.
Article30. Laging M, Kal-van Gestel JA, Haasnoot GW, Claas FH, van de Wetering J, Ijzermans JN, et al. 2014; Transplantation results of completely HLA-mismatched living and completely HLA-matched deceased-donor kidneys are comparable. Transplantation. 97:330–6. DOI: 10.1097/01.TP.0000435703.61642.43. PMID: 24202143.
Article31. Roodnat JI, van Riemsdijk IC, Mulder PGH, Doxiadis I, Claas FHJ, IJzermans JNM, et al. 2003; The superior results of living-donor renal transplantation are not completely caused by selection or short cold ischemia time: a single-center, multivariate analysis. Transplantation. 75:2014–8. DOI: 10.1097/01.TP.0000065176.06275.42. PMID: 12829903.
Article32. Koo DD, Welsh KI, McLaren AJ, Roake JA, Morris PJ, Fuggle SV. 1999; Cadaver versus living donor kidneys: impact of donor factors on antigen induction before transplantation. Kidney Int. 56:1551–9. DOI: 10.1046/j.1523-1755.1999.00657.x. PMID: 10504507.
Article33. Zhang R. 2018; Donor-specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol. 13:182–92. DOI: 10.2215/CJN.00700117. PMID: 28446536. PMCID: PMC5753302.
Article34. Hirai T, Kohei N, Omoto K, Ishida H, Tanabe K. 2012; Significance of low-level DSA detected by solid-phase assay in association with acute and chronic antibody-mediated rejection. Transpl Int. 25:925–34. DOI: 10.1111/j.1432-2277.2012.01518.x. PMID: 22764746.
Article35. Willicombe M, Brookes P, Santos-Nunez E, Galliford J, Ballow A, McLean A, et al. 2011; Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 11:470–7. DOI: 10.1111/j.1600-6143.2010.03421.x. PMID: 21299828.
Article36. Aubert O, Loupy A, Hidalgo L, Duong van Huyen JP, Higgins S, Viglietti D, et al. 2017; Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol. 28:1912–23. DOI: 10.1681/ASN.2016070797. PMID: 28255002. PMCID: PMC5461792.
Article37. Park WY, Kim Y, Paek JH, Jin K, Han S. 2021; Clinical significance of de novo donor-specific antibody in kidney transplant recipients with chronic antibody-mediated rejection. Korean J Transplant. 35:33–40. DOI: 10.4285/kjt.20.0052. PMID: 35769616. PMCID: PMC9235340.
Article38. Montgomery RA, Tatapudi VS, Leffell MS, Zachary AA. 2018; HLA in transplantation. Nat Rev Nephrol. 14:558–70. DOI: 10.1038/s41581-018-0039-x. PMID: 29985463.
Article39. Avery RK, Motter JD, Jackson KR, Montgomery RA, Massie AB, Kraus ES, et al. 2021; Quantifying infection risks in incompatible living donor kidney transplant recipients. Am J Transplant. 21:1564–75. DOI: 10.1111/ajt.16316. PMID: 32949093. PMCID: PMC7972996.
Article40. Chung BH, Yun JT, Ha SE, Kim JI, Moon IS, Choi BS, et al. 2013; Combined use of rituximab and plasmapheresis pre-transplant increases post-transplant infections in renal transplant recipients with basiliximab induction therapy. Transpl Infect Dis. 15:559–68. DOI: 10.1111/tid.12135. PMID: 24011062.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of preexisting human leukocyte antigen donor-specific antibodies especially human leukocyte antigen-DQ on kidney transplant outcome
- Impact of low-level donor-specific anti-HLA antibody on posttransplant clinical outcomes of kidney transplant recipients: analysis from Korean Organ Transplantation Registry data
- The clinical impact of preformed human leukocyte antigen-DQ donor-specific antibodies on graft outcomes in kidney transplantation
- Clinical relevance and characteristics of pretransplant donor-specific anti-human leukocyte antigen-DQ antibodies in kidney transplantation
- Clinical significance of donor-specific anti-HLA-DR51/52/53 antibodies for antibody-mediated rejection in kidney transplant recipients