J Stroke.
2024 Jan;26(1):26-40. 10.5853/jos.2023.02481.
Emerging Concept of Intracranial Arterial Diseases: The Role of High Resolution Vessel Wall MRI
- Affiliations
-
- 1Department of Neurology, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Headquarters for Public Health Care, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Neurology, Gyeonggi Provincial Medical Center, Icheon Hospital, Icheon, Korea
- 4Department of Neurology, Inha University Hospital, Incheon, Korea
- 5Department of Radiology, Seoul National University Bundang Hospital, Seongnam, Korea
- 6Department of Internal Medicine-Neurology Division, Max Rady College of Medicine, University of Manitoba, Winnipeg, MB, Canada
- 7Department of Radiology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA
- KMID: 2551345
- DOI: http://doi.org/10.5853/jos.2023.02481
Abstract
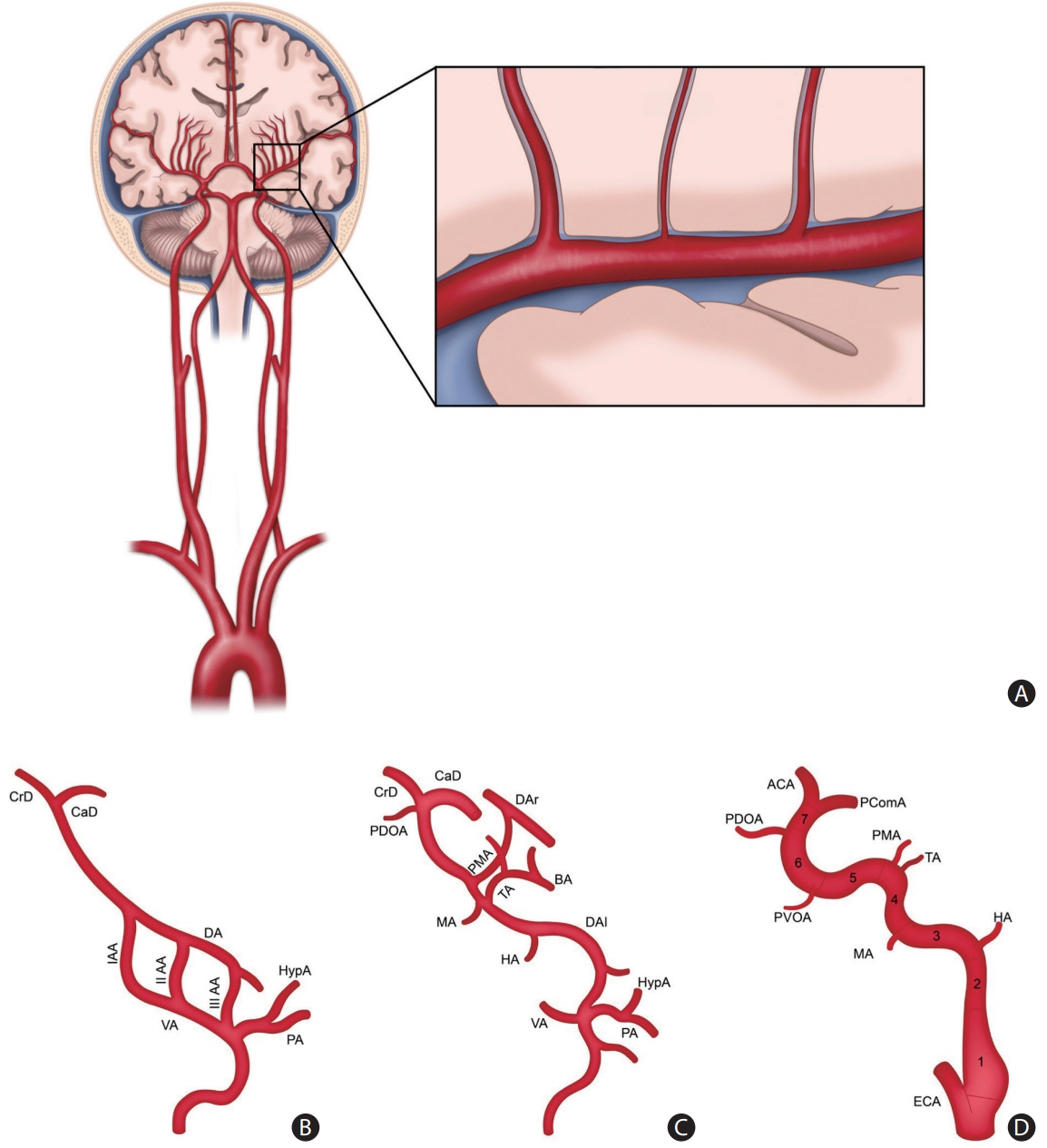
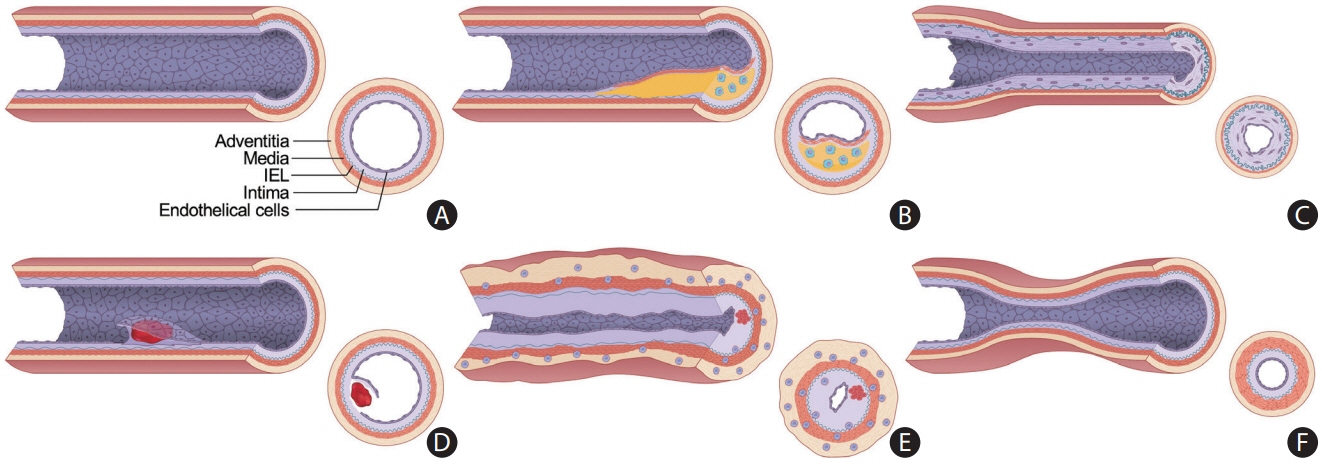
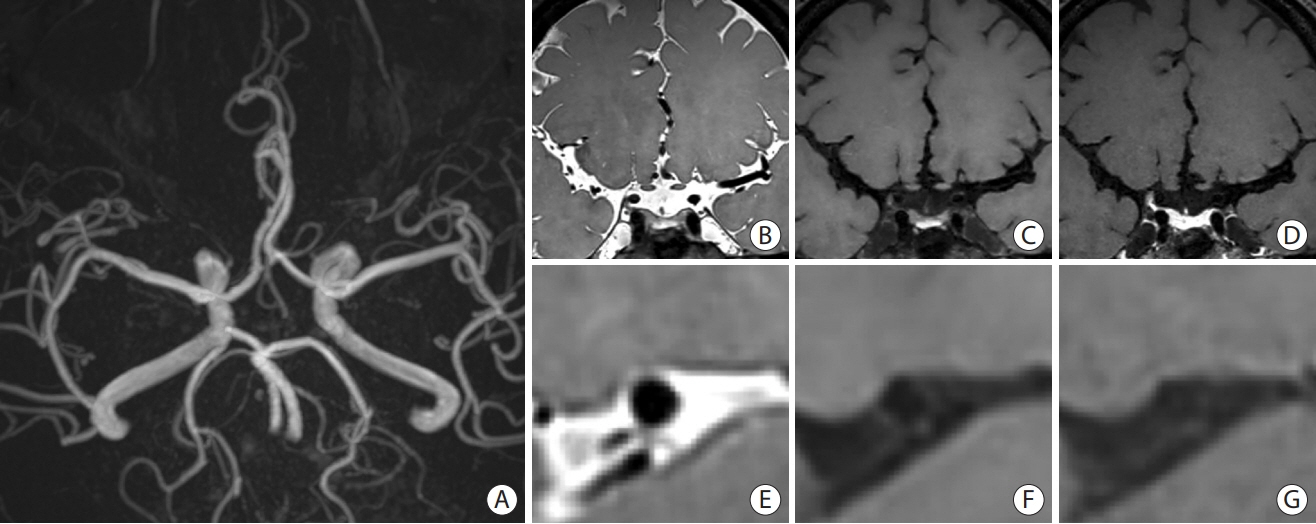
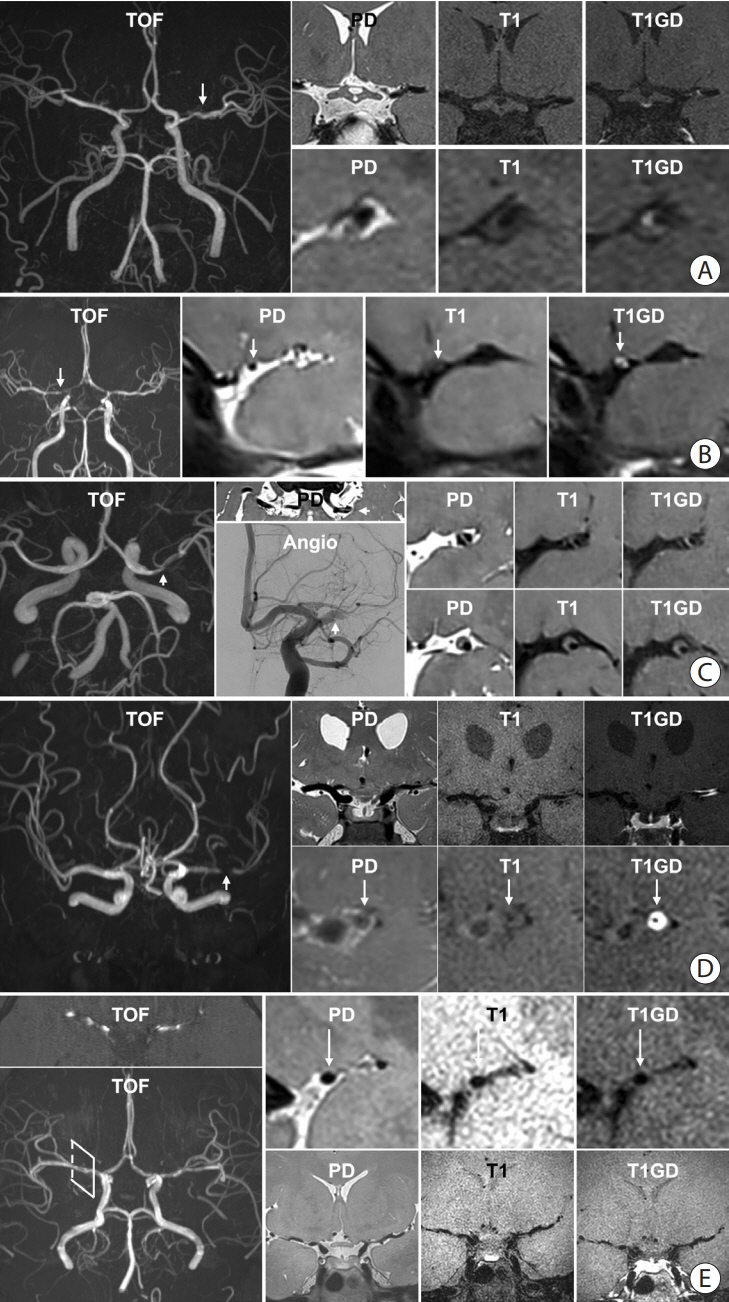
- Intracranial arterial disease (ICAD) is a heterogeneous condition characterized by distinct pathologies, including atherosclerosis. Advances in magnetic resonance technology have enabled the visualization of intracranial arteries using high-resolution vessel wall imaging (HR-VWI). This review summarizes the anatomical, embryological, and histological differences between the intracranial and extracranial arteries. Next, we review the heterogeneous pathophysiology of ICAD, including atherosclerosis, moyamoya or RNF213 spectrum disease, intracranial dissection, and vasculitis. We also discuss how advances in HR-VWI can be used to differentiate ICAD etiologies. We emphasize that one should consider clinical presentation and timing of imaging in the absence of pathology-radiology correlation data. Future research should focus on understanding the temporal profile of HR-VWI findings and developing quantitative interpretative approaches to improve the decision-making and management of ICAD.
Keyword
Figure
Cited by 1 articles
-
Narrowings of the Deep Cerebral Perforating Arteries Ostia: Geometry, Structure, and Clinical Implications
Radosław Rzepliński, Sylwia Tarka, Michał Tomaszewski, Michał Kucewicz, Albert Acewicz, Jerzy Małachowski, Bogdan Ciszek
J Stroke. 2025;27(1):52-64. doi: 10.5853/jos.2024.01655.
Reference
-
References
1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008; 39:2396–2399.2. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014; 383:333–341.3. Flusty B, de Havenon A, Prabhakaran S, Liebeskind DS, Yaghi S. Intracranial atherosclerosis treatment: past, present, and future. Stroke. 2020; 51:e49–e53.4. Bala F, Cimflova P, Singh N, Zhang J, Kappelhof M, Kim BJ, et al. Impact of vessel tortuosity and radiological thrombus characteristics on the choice of first-line thrombectomy strategy: results from the ESCAPE-NA1 trial. Eur Stroke J. 2023; 8:675–683.5. Bang OY, Toyoda K, Arenillas JF, Liu L, Kim JS. Intracranial large artery disease of non-atherosclerotic origin: recent progress and clinical implications. J Stroke. 2018; 20:208–217.6. de Havenon A, Mossa-Basha M, Shah L, Kim SE, Park M, Parker D, et al. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology. 2017; 59:1193–1202.7. Kim JS, Caplan LR. Non-atherosclerotic intracranial arterial diseases. Front Neurol Neurosci. 2016; 40:179–203.8. Lasjaunias PL. Segmental identity and vulnerability in cerebral arteries. Interv Neuroradiol. 2000; 6:113–124.9. Namba K. Carotid-vertebrobasilar anastomoses with reference to their segmental property. Neurol Med Chir (Tokyo). 2017; 57:267–277.10. Bevan JA. Sites of transition between functional systemic and cerebral arteries of rabbits occur at embryological junctional sites. Science. 1979; 204:635–637.11. Komiyama M. Segmental vulnerability and vascular neurocristopathy of the internal carotid artery. Interv Neuroradiol. 2020; 26:131–134.12. Kobkitsuksakul C, Somboonnitiphol K, Apirakkan M, Lueangapapong P, Chanthanaphak E. Dolichoectasia of the internal carotid artery terminus, posterior communicating artery, and posterior cerebral artery: the embryonic caudal ramus internal carotid segmental vulnerability legacy. Interv Neuroradiol. 2020; 26:124–130.13. Yang WJ, Wong KS, Chen XY. Intracranial atherosclerosis: from microscopy to high-resolution magnetic resonance imaging. J Stroke. 2017; 19:249–260.14. Zervas NT, Liszczak TM, Mayberg MR, Black PM. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg. 1982; 56:475–481.15. Velican C, Velican D. Atherosclerotic involvement of human intracranial arteries with special reference to intimal necrosis. Atherosclerosis. 1982; 43:59–69.16. Portanova A, Hakakian N, Mikulis DJ, Virmani R, Abdalla WM, Wasserman BA. Intracranial vasa vasorum: insights and implications for imaging. Radiology. 2013; 267:667–679.17. Williams JK, Heistad DD. Structure and function of vasa vasorum. Trends Cardiovasc Med. 1996; 6:53–57.18. Bae HJ, Lee J, Park JM, Kwon O, Koo JS, Kim BK, et al. Risk factors of intracranial cerebral atherosclerosis among asymptomatics. Cerebrovasc Dis. 2007; 24:355–360.19. Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014; 130:1407–1414.20. Chen PC, Yang SH, Chien KL, Tsai IJ, Kuo MF. Epidemiology of moyamoya disease in Taiwan: a nationwide population-based study. Stroke. 2014; 45:1258–1263.21. Kim T, Lee H, Bang JS, Kwon OK, Hwang G, Oh CW. Epidemiology of moyamoya disease in Korea: based on National Health Insurance Service data. J Korean Neurosurg Soc. 2015; 57:390–395.22. Kuroda S, Fujimura M, Takahashi J, Kataoka H, Ogasawara K, Iwama T, et al. Diagnostic criteria for moyamoya disease -2021 revised version. Neurol Med Chir (Tokyo). 2022; 62:307–312.23. Ihara M, Yamamoto Y, Hattori Y, Liu W, Kobayashi H, Ishiyama H, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. 2022; 21:747–758.24. Liu W, Hitomi T, Kobayashi H, Harada KH, Koizumi A. Distribution of moyamoya disease susceptibility polymorphism p.R4810K in RNF213 in East and Southeast Asian populations. Neurol Med Chir (Tokyo). 2012; 52:299–303.25. Kim HJ, Choi EH, Chung JW, Kim JH, Kim YS, Seo WK, et al. Role of the RNF213 variant in vascular outcomes in patients with intracranial atherosclerosis. J Am Heart Assoc. 2021; 10:e017660.26. Bang OY, Chung JW, Kim DH, Won HH, Yeon JY, Ki CS, et al. Moyamoya disease and spectrums of RNF213 vasculopathy. Transl Stroke Res. 2020; 11:580–589.27. Ge P, Ye X, Liu X, Deng X, Wang R, Zhang Y, et al. Association between p.R4810K variant and long-term clinical outcome in patients with moyamoya disease. Front Neurol. 2019; 10:662.28. Kim JS, Lee HB, Kwon HS. RNF213 polymorphism in intracranial artery dissection. J Stroke. 2018; 20:404–406.29. Ok T, Jung YH, Lee KY. Genotype-phenotype correlation of the RNF213 R4810K variant in moyamoya disease. J Stroke. 2023; 25:303–306.30. Park MS, Cha J, Chung JW, Seo WK, Kim GM, Bang OY. Arterial dissection as a cause of intracranial stenosis in East Asians. J Am Coll Cardiol. 2017; 70:2205–2206.31. Ahn SS, Kim BM, Suh SH, Kim DJ, Kim DI, Shin YS, et al. Spontaneous symptomatic intracranial vertebrobasilar dissection: initial and follow-up imaging findings. Radiology. 2012; 264:196–202.32. Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y, et al. Symptomatic recurrence of intracranial arterial dissections: follow-up study of 143 consecutive cases and pathological investigation. Stroke. 2013; 44:126–131.33. Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015; 14:640–654.34. Rice CM, Scolding NJ. The diagnosis of primary central nervous system vasculitis. Pract Neurol. 2020; 20:109–114.35. Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol. 1995; 22:662–667.36. Cho S, Kim J, Park M, Lee KY. High-resolution vessel wall magnetic resonance imaging of varicella-zoster virus vasculopathy affecting the vertebrobasilar artery. J Neurosonol Neuroimag. 2020; 12:51–54.37. Patzig M, Forbrig R, Küpper C, Eren O, Saam T, Kellert L, et al. Diagnosis and follow-up evaluation of central nervous system vasculitis: an evaluation of vessel-wall MRI findings. J Neurol. 2022; 269:982–996.38. Shimoyama T, Uchino K, Calabrese LH, Hajj-Ali RA. Serial vessel wall enhancement pattern on high-resolution vessel wall magnetic resonance imaging and clinical implications in patients with central nervous system vasculitis. Clin Exp Rheumatol. 2022; 40:811–818.39. Song JW, Lehman L, Rivkin M, Gorman MP, Yang E. Serial vessel wall MR imaging of pediatric tuberculous vasculitis. Neurol Clin Pract. 2019; 9:459–461.40. Alexander MD, Yuan C, Rutman A, Tirschwell DL, Palagallo G, Gandhi D, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry. 2016; 87:589–597.41. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2017; 38:218–229.42. Kim HJ, Choi EH, Chung JW, Kim JH, Kim YS, Seo WK, et al. Luminal and wall changes in intracranial arterial lesions for predicting stroke occurrence. Stroke. 2020; 51:2495–2504.43. Lehman VT, Brinjikji W, Kallmes DF, Huston J 3rd, Lanzino G, Rabinstein AA, et al. Clinical interpretation of high-resolution vessel wall MRI of intracranial arterial diseases. Br J Radiol. 2016; 89:20160496.44. Zhang L, Zhang N, Wu J, Zhang L, Huang Y, Liu X, et al. High resolution three dimensional intracranial arterial wall imaging at 3 T using T1 weighted SPACE. Magn Reson Imaging. 2015; 33:1026–1034.45. Mazzacane F, Mazzoleni V, Scola E, Mancini S, Lombardo I, Busto G, et al. Vessel wall magnetic resonance imaging in cerebrovascular diseases. Diagnostics (Basel). 2022; 12:258.46. Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology. 2018; 286:12–28.47. Kang N, Qiao Y, Wasserman BA. Essentials for interpreting intracranial vessel wall MRI results: state of the art. Radiology. 2021; 300:492–505.48. San Millán Ruíz D, Gailloud P, Rüfenacht DA, Delavelle J, Henry F, Fasel JH. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol. 2002; 23:1500–1508.49. Kim DJ, Lee HJ, Baik J, Hwang MJ, Miyoshi M, Kang Y. Improved blood suppression of motion-sensitized driven equilibrium in high-resolution whole-brain vessel wall imaging: comparison of contrast-enhanced 3D T1-weighted FSE with motion-sensitized driven equilibrium and delay alternating with nutation for tailored excitation. AJNR Am J Neuroradiol. 2022; 43:1713–1718.50. Hu X, Li Y, Zhang L, Zhang X, Liu X, Chung YC. A 32-channel coil system for MR vessel wall imaging of intracranial and extracranial arteries at 3T. Magn Reson Imaging. 2017; 36:86–92.51. Busse RF, Brau AC, Vu A, Michelich CR, Bayram E, Kijowski R, et al. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med. 2008; 60:640–649.52. Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 tesla. J Magn Reson Imaging. 2011; 34:22–30.53. Guggenberger KV, Torre GD, Ludwig U, Vogel P, Weng AM, Vogt ML, et al. Vasa vasorum of proximal cerebral arteries after dural crossing - potential imaging confounder in diagnosing intracranial vasculitis in elderly subjects on black-blood MRI. Eur Radiol. 2022; 32:1276–1284.54. Kasab SA, Bathla G, Varon A, Roa JA, Sabotin R, Raghuram A, et al. High-resolution vessel wall imaging after mechanical thrombectomy. Neuroradiol J. 2021; 34:593–599.55. Chung JW, Bang OY, Lee MJ, Hwang J, Cha J, Choi JH, et al. Echoing plaque activity of the coronary and intracranial arteries in patients with stroke. Stroke. 2016; 47:1527–1533.56. Kim JY, Kim HJ, Choi EH, Pan KH, Chung JW, Seo WK, et al. Vessel wall changes on serial high-resolution MRI and the use of cilostazol in patients with adult-onset moyamoya disease. J Clin Neurol. 2022; 18:610–618.57. Cheng W, Xue S, Wu F, Song X, Huang Q, Song H, et al. The clinical and vascular characteristics of RNF213 c.14576G>A variant-related intracranial major artery disease in China. Behav Neurol. 2019; 2019:7908392.58. Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012; 11:906–917.59. Mossa-Basha M, Shibata DK, Hallam DK, de Havenon A, Hippe DS, Becker KJ, et al. Added value of vessel wall magnetic resonance imaging for differentiation of nonocclusive intracranial vasculopathies. Stroke. 2017; 48:3026–3033.60. Shen ZZ, Ren SJ, Wu RR, Su CQ, Ge S, Hong XN, et al. Temporal changes in plaque characteristics after treatment and their relationship with stroke recurrence: a quantitative study using magnetic resonance imaging. Quant Imaging Med Surg. 2022; 12:4559–4569.61. Kwee RM, Qiao Y, Liu L, Zeiler SR, Wasserman BA. Temporal course and implications of intracranial atherosclerotic plaque enhancement on high-resolution vessel wall MRI. Neuroradiology. 2019; 61:651–657.62. Chung JW, Cha J, Lee MJ, Yu IW, Park MS, Seo WK, et al. Intensive statin treatment in acute ischaemic stroke patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study (STAMINA-MRI Study). J Neurol Neurosurg Psychiatry. 2020; 91:204–211.63. Lin X, Guo W, She D, Wang F, Xing Z, Cao D. Follow-up assessment of atherosclerotic plaques in acute ischemic stroke patients using high-resolution vessel wall MR imaging. Neuroradiology. 2022; 64:2257–2266.64. Meng Y, Zhang Y, Chu X, Song Y, Zhao W, Zheng M, et al. Plaque modification and stabilization after drug-coated balloon angioplasty for intracranial atherosclerotic lesions. Eur Radiol. 2023; 33:1112–1120.65. Wu CH, Chung CP, Chen TY, Yu KW, Lin TM, Tai WA, et al. Influence of angioplasty and stenting on intracranial artery stenosis: preliminary results of high-resolution vessel wall imaging evaluation. Eur Radiol. 2022; 32:6788–6799.66. Lee SH, Kim KY, Jung JM. High-resolution magnetic resonance imaging for the follow-up of intracranial arterial dissections. Acta Neurol Belg. 2021; 121:1599–1605.67. Lu M, Zhang H, Liu D, Hao F, Zhang L, Peng P, et al. Vessel wall enhancement as a predictor of arterial stenosis progression and poor outcomes in moyamoya disease. Eur Radiol. 2023; 33:2489–2499.68. Yeo J, Hwang I, Sohn CH, Lee EE, Lee ST, Lee EB, et al. Proliferative vasculopathy associated with antiphospholipid antibodies in patients with neurological symptoms. Front Med (Lausanne). 2022; 9:913203.69. Choi EH, Yu I, Park JH, Yoon CW, Bang OY. Reversible cerebral vasoconstriction syndrome without typical thunderclap headache: highresolution magnetic resonance imaging features. Precis Future Med. 2018; 2:175–179.70. Burton TM, Bushnell CD. Reversible cerebral vasoconstriction syndrome. Stroke. 2019; 50:2253–2258.71. Shi Z, Li J, Zhao M, Zhang X, Degnan AJ, Mossa-Basha M, et al. Progression of plaque burden of intracranial atherosclerotic plaque predicts recurrent stroke/transient ischemic attack: a pilot follow-up study using higher-resolution MRI. J Magn Reson Imaging. 2021; 54:560–570.72. Lee HS, Jung JM, Yang HB, Lee SH. Predicting stenosis aggravation in follow-up high-resolution magnetic resonance images of patients with intracranial atherosclerosis. Cerebrovasc Dis. 2022; 51:608–614.73. Zhang S, Wang J, Lu J, Qi P, Hu S, Yang X, et al. Plaque characteristics after endovascular treatment in patients with intracranial atherosclerotic disease. Chin Neurosurg J. 2022; 8:37.74. Xiao J, Song SS, Schlick KH, Xia S, Jiang T, Han T, et al. Disparate trends of atherosclerotic plaque evolution in stroke patients under 18-month follow-up: a 3D whole-brain magnetic resonance vessel wall imaging study. Neuroradiol J. 2022; 35:42–52.75. Huang J, Jiao S, Chen Y, Lu J, Song Y, Zhang J, et al. Efficacy of medical treatment and balloon angioplasty for severe intracranial atherosclerosis: a high-resolution MR vessel wall imaging. Eur Radiol. 2023; 33:2478–2488.76. Kwon H, Jung SC, Young CJ, Kang DW, Kwon SU, Kim JS, et al. Structural changes of intra and extracranial artery dissection: a study of high-resolution magnetic resonance imaging. J Stroke Cerebrovasc Dis. 2022; 31:106302.77. Kang H, Bai X, Zhang Y, Zhou W, Ju Y, Yang X, et al. Predictors of improvement for patients with CNS vasculitis stenoses: a high-resolution vessel wall MRI follow-up study. Eur J Radiol. 2023; 158:110619.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vessel Wall Imaging of the Intracranial and Cervical Carotid Arteries
- High-Resolution Magnetic Resonance Imaging of Intracranial Vertebral Artery Dissecting Aneurysm for Planning of Endovascular Treatment
- Cerebral Infarction Secondary to Vascular Infiltration of Glioblastoma Confirmed in High-Resolution Vessel Wall MRI
- Isolated Posteroinferior Cerebellar Artery Dissection Diagnosed by High-Resolution Vessel Wall MRI
- High-Resolution MRI of Intracranial Atherosclerotic Disease