Diabetes Metab J.
2024 Jan;48(1):146-156. 10.4093/dmj.2022.0100.
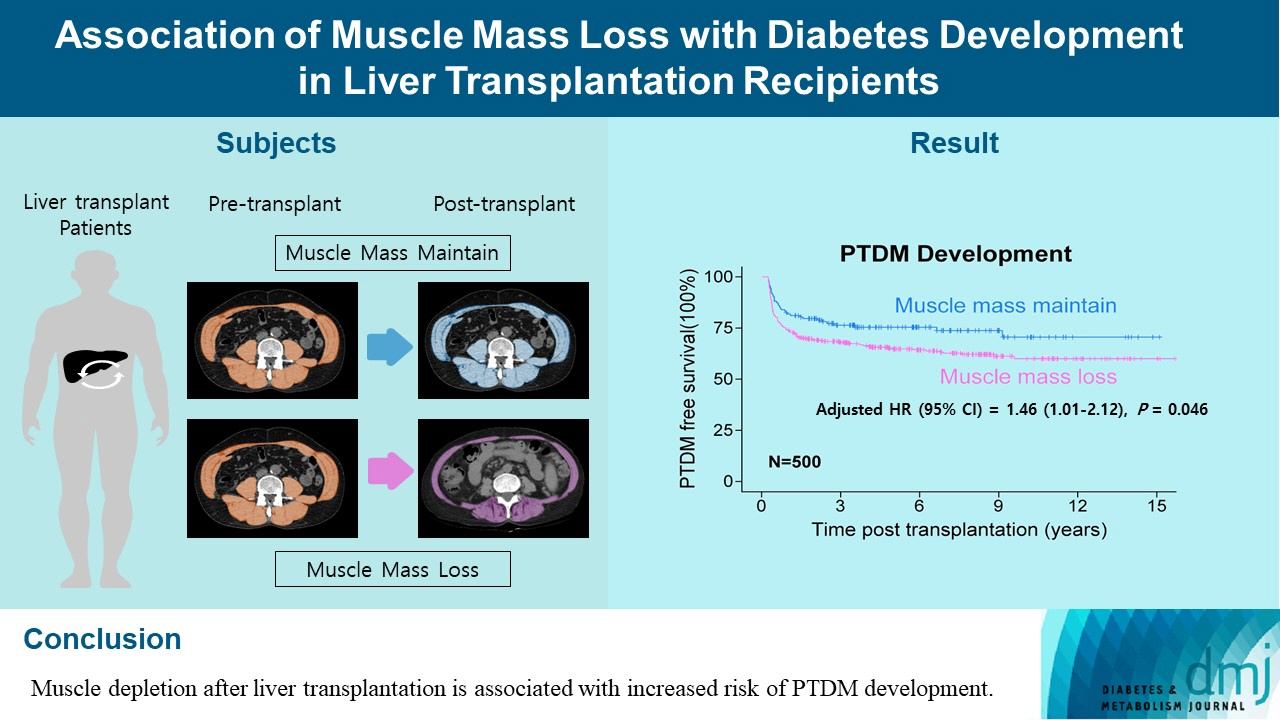
Association of Muscle Mass Loss with Diabetes Development in Liver Transplantation Recipients
- Affiliations
-
- 1Graduate School, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Pharmacology, Graduate School of Medical Science, Brain Korea 21 Project, Yonsei University College of Medicine, Seoul, Korea
- 5Mo-Im Kim Nursing Research Institute, Biobehavioral Research Center, Yonsei University College of Nursing, Seoul, Korea
- 6Division of Transplantation, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2551270
- DOI: http://doi.org/10.4093/dmj.2022.0100
Abstract
- Background
Post-transplant diabetes mellitus (PTDM) is one of the most significant complications after transplantation. Patients with end-stage liver diseases requiring transplantation are prone to sarcopenia, but the association between sarcopenia and PTDM remains to be elucidated. We aimed to investigate the effect of postoperative muscle mass loss on PTDM development.
Methods
A total of 500 patients who underwent liver transplantation at a tertiary care hospital between 2005 and 2020 were included. Skeletal muscle area at the level of the L3–L5 vertebrae was measured using computed tomography scans performed before and 1 year after the transplantation. The associations between the change in the muscle area after the transplantation and the incidence of PTDM was investigated using a Cox proportional hazard model.
Results
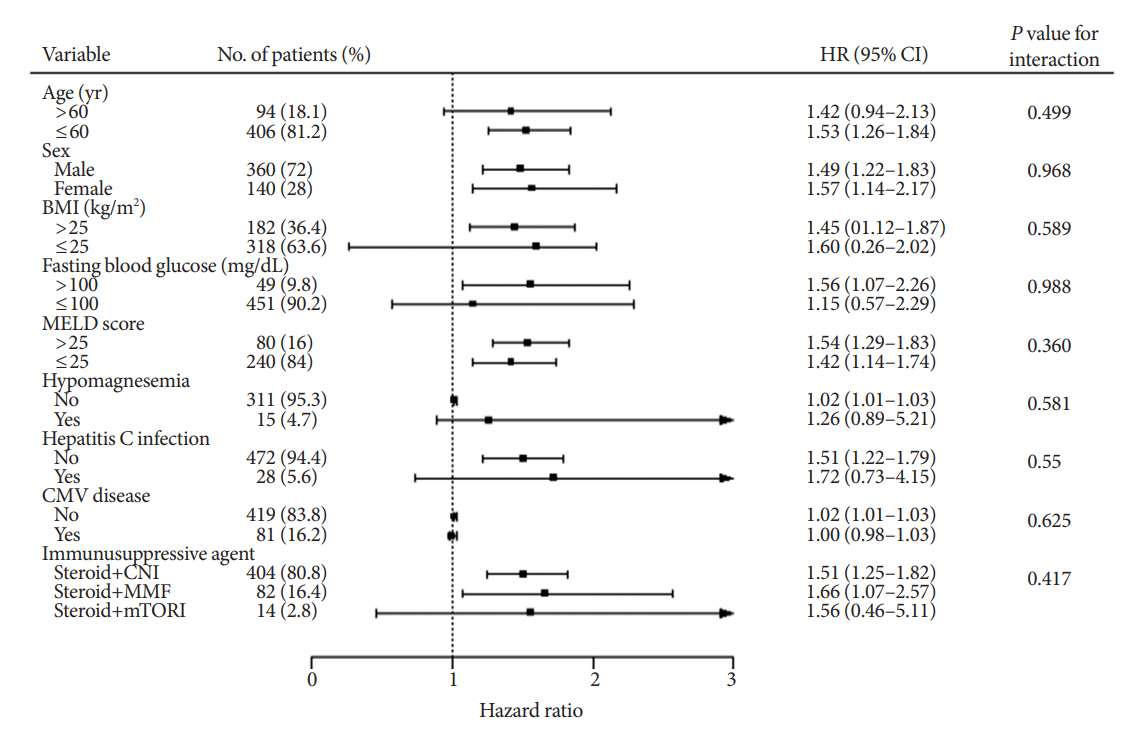
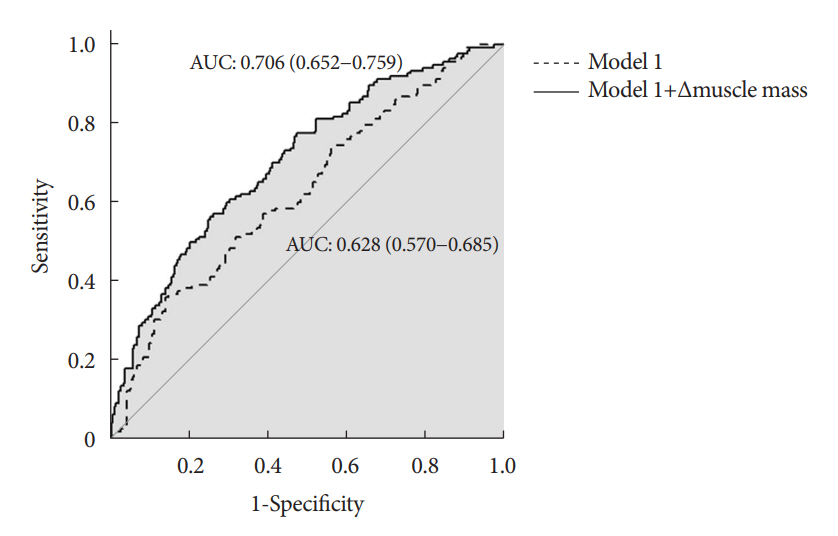
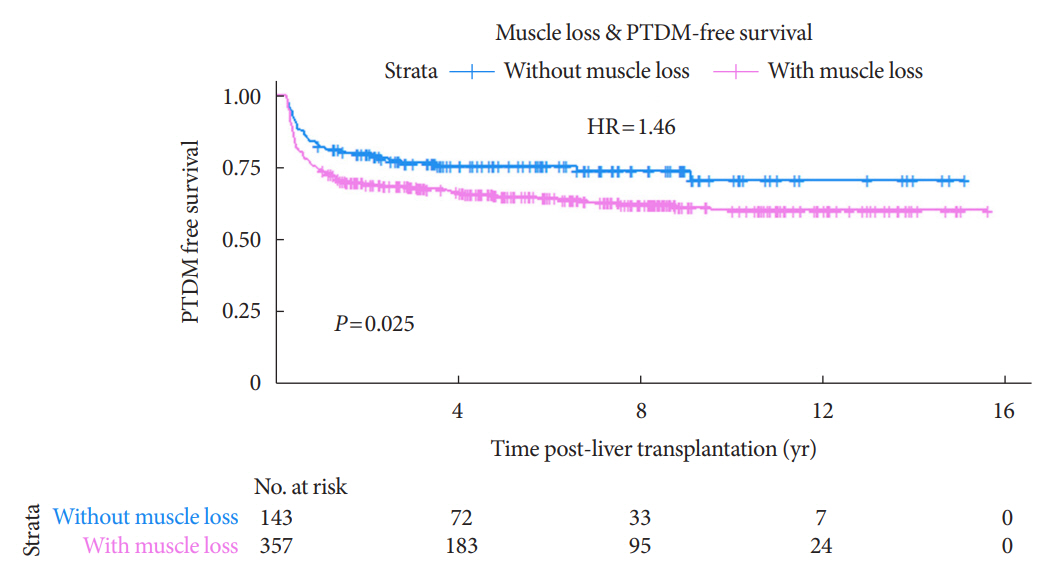
During the follow-up period (median, 4.9 years), PTDM occurred in 165 patients (33%). The muscle mass loss was greater in patients who developed PTDM than in those without PTDM. Muscle depletion significantly increased risk of developing PTDM after adjustment for other confounding factors (hazard ratio, 1.50; 95% confidence interval, 1.23 to 1.84; P=0.001). Of the 357 subjects who had muscle mass loss, 124 (34.7%) developed PTDM, whereas of the 143 patients in the muscle mass maintenance group, 41 (28.7%) developed PTDM. The cumulative incidence of PTDM was significantly higher in patients with muscle loss than in patients without muscle loss (P=0.034).
Conclusion
Muscle depletion after liver transplantation is associated with increased risk of PTDM development.
Figure
Reference
-
1. Rana A, Gruessner A, Agopian VG, Khalpey Z, Riaz IB, Kaplan B, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015; 150:252–9.2. Friedman AN, Miskulin DC, Rosenberg IH, Levey AS. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis. 2003; 41:480–7.3. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003; 3:178–85.4. Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019; 15:172–88.5. Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013; 58:3103–11.6. Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis: aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016; 43:765–77.7. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017; 12:e0186990.8. Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019; 48:48–56.9. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019; 12:1057–72.10. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr J. 2014; 61:61–70.11. Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014; 20:640–8.12. Pinto Dos Santos D, Kloeckner R, Koch S, Hoppe-Lotichius M, Zoller D, Toenges G, et al. Sarcopenia as prognostic factor for survival after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2020; 32:626–34.13. Chang KV, Chen JD, Wu WT, Huang KC, Han DS. Association of loss of muscle mass with mortality in liver cirrhosis without or before liver transplantation: a systematic review and meta-analysis. Medicine (Baltimore). 2019; 98:e14373.14. Kuo SZ, Ahmad M, Dunn MA, Montano-Loza AJ, Carey EJ, Lin S, et al. Sarcopenia predicts post-transplant mortality in acutely ill men undergoing urgent evaluation and liver transplantation. Transplantation. 2019; 103:2312–7.15. Dasarathy S. Myostatin and beyond in cirrhosis: all roads lead to sarcopenia. J Cachexia Sarcopenia Muscle. 2017; 8:864–9.16. Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014; 29:1250–7.17. Yadav AD, Chang YH, Aqel BA, Byrne TJ, Chakkera HA, Douglas DD, et al. New onset diabetes mellitus in living donor versus deceased donor liver transplant recipients: analysis of the UNOS/OPTN Database. J Transplant. 2013; 2013:269096.18. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37 Suppl 1:S81–90.19. Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014; 14:1992–2000.20. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39:412–23.21. Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004; 97:2333–8.22. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998; 85:115–22.23. Xie L, Tang W, Wang X, Wang L, Lu Y, Lin T. Pretransplantation risk factors associated with new-onset diabetes after living-donor kidney transplantation. Transplant Proc. 2016; 48:3299–302.24. Han E, Kim MS, Kim YS, Kang ES. Risk assessment and management of post-transplant diabetes mellitus. Metabolism. 2016; 65:1559–69.25. An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, et al. Sarcopenia in autoimmune and rheumatic diseases: a comprehensive review. Int J Mol Sci. 2020; 21:5678.26. Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019; 70:1816–29.27. Werzowa J, Pacini G, Hecking M, Fidler C, Haidinger M, Brath H, et al. Comparison of glycemic control and variability in patients with type 2 and posttransplantation diabetes mellitus. J Diabetes Complications. 2015; 29:1211–6.28. Valderhaug TG, Hjelmesæth J, Hartmann A, Roislien J, Bergrem HA, Leivestad T, et al. The association of early posttransplant glucose levels with long-term mortality. Diabetologia. 2011; 54:1341–9.29. Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021; 74:1611–44.30. Hussaini SH, Oldroyd B, Stewart SP, Soo S, Roman F, Smith MA, et al. Effects of orthotopic liver transplantation on body composition. Liver. 1998; 18:173–9.31. Plank LD, Metzger DJ, McCall JL, Barclay KL, Gane EJ, Streat SJ, et al. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann Surg. 2001; 234:245–55.32. Muller MJ, Loyal S, Schwarze M, Lobers J, Selberg O, Ringe B, et al. Resting energy expenditure and nutritional state in patients with liver cirrhosis before and after liver transplantation. Clin Nutr. 1994; 13:145–52.33. Kallwitz ER, Loy V, Mettu P, Von Roenn N, Berkes J, Cotler SJ. Physical activity and metabolic syndrome in liver transplant recipients. Liver Transpl. 2013; 19:1125–31.34. Kallwitz ER. Sarcopenia and liver transplant: the relevance of too little muscle mass. World J Gastroenterol. 2015; 21:10982–93.35. Kivela R, Salmela I, Nguyen YH, Petrova TV, Koistinen HA, Wiener Z, et al. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat Commun. 2016; 7:13124.36. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016; 32:1200–5.37. Fosbol MO, Zerahn B. Contemporary methods of body composition measurement. Clin Physiol Funct Imaging. 2015; 35:81–97.38. European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016; 64:433–85.39. Uchiyama H. Sarcopenia in liver transplant recipients: its relevance to peritransplant morbidity and mortality. Hepatobiliary Surg Nutr. 2017; 6:196–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Tuberculosis in Liver or Heart Transplant Recipients
- Association of pretransplant skeletal muscle mass with outcomes in kidney transplant recipients
- Posttransplant Diabetes Mellitus after Liver Transplantation: Risk Factors for Persistence
- Split liver transplantation for one adult and one pediatric recipient: A collective review of Korean experience
- Indication of Pediatric Liver Transplantation