J Korean Med Sci.
2024 Jan;39(3):e15. 10.3346/jkms.2024.39.e15.
Effectiveness of Bivalent mRNA Booster Vaccine Against COVID-19 in Korea
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Vaccine Innovation Center-KU Medicine (VIC-K), Seoul, Korea
- 3Division of Infectious Diseases, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 4Division of Infectious Diseases, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 5Division of Infectious Diseases, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 6Division of Infectious Diseases, Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 7Division of Infectious Diseases, Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea
- 8Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea
- 9Division of Infectious Diseases, Department of Internal Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- KMID: 2551175
- DOI: http://doi.org/10.3346/jkms.2024.39.e15
Abstract
- Background
Bivalent booster mRNA vaccines containing the omicron-variant strains have been introduced worldwide in the autumn of 2022. Nevertheless, the omicron subvariants evoked another large coronavirus disease 2019 (COVID-19) pandemic wave in late 2022 and early 2023.
Methods
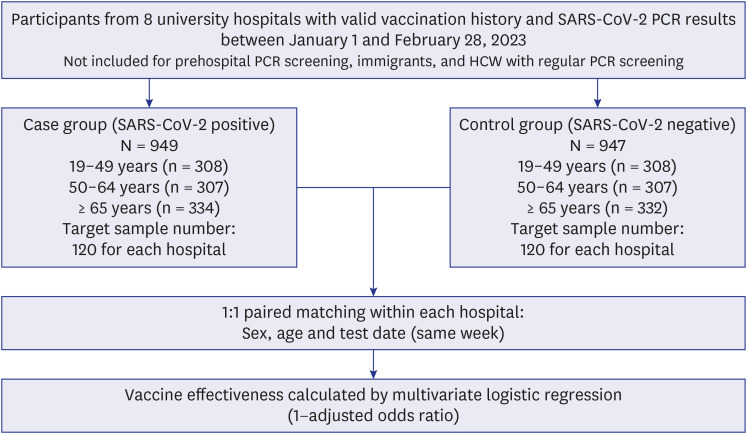
A retrospective, test-negative, case-control study was conducted to estimate the vaccine effectiveness (VE) of bivalent COVID-19 vaccines in 8 university hospitals between January and February 2023. The case and control groups were divided based on nasopharyngeal COVID-19 real-time polymerase chain reaction results and matched based on age, sex, hospital, and date (week) of the test performed. The VE of the BA.1- or BA.4/BA.5-based mRNA vaccines were estimated. VE was calculated using the 1−adjusted odds ratio from multivariable logistic regression.
Results
In total, 949 patients and 947 controls were enrolled in this study. VE for the BA.4/ BA.5-based bivalent mRNA vaccine was 43% (95% confidence interval [CI], 17, 61%). In subgroup analysis based on age and underlying medical conditions, BA.4/BA.5-based bivalent mRNA vaccine was effective against old adults aged ≥ 65-years (VE, 55%; 95% CI, 23, 73%) and individuals with comorbidities (VE, 54%; 95% CI, 23, 73%). In comparison, the BA.1-based bivalent mRNA vaccine did not demonstrate statistically significant effectiveness (VE, 25%; 95% CI, −8, 49%).
Conclusion
The BA.4/BA.5-based bivalent mRNA booster vaccine provided significant protection against COVID-19 in the Korean adults, especially in the older adults aged ≥ 65 years and in individuals with underlying medical conditions.
Figure
Reference
-
1. Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022; 386(4):340–350. PMID: 35021002.2. Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021; 385(24):e83. PMID: 34614327.3. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022; 399(10328):924–944. PMID: 35202601.4. World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Updated 2021. Accessed August 30, 2023. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern .5. Ai J, Zhang H, Zhang Y, Lin K, Zhang Y, Wu J, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022; 11(1):337–343. PMID: 34935594.6. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID: 35249272.7. World Health Organization. Evaluation of COVID-19 vaccine effectiveness: interim guidance, 17 March 2021. Updated 2021. Accessed August 30, 2023. https://apps.who.int/iris/handle/10665/340301 .8. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370(9596):1453–1457. PMID: 18064739.9. Tseng HF, Ackerson BK, Bruxvoort KJ, Sy LS, Tubert JE, Lee GS, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 Omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun. 2023; 14(1):189. PMID: 36635284.10. Tenforde MW, Weber ZA, Natarajan K, Klein NP, Kharbanda AB, Stenehjem E, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated emergency department or urgent care encounters and hospitalizations among immunocompetent adults - vision network, nine states, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(5152):1616–1624. PMID: 36580430.11. Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, et al. A bivalent omicron-containing booster vaccine against COVID-19. N Engl J Med. 2022; 387(14):1279–1291. PMID: 36112399.12. Shrestha NK, Burke PC, Nowacki AS, Simon JF, Hagen A, Gordon SM. Effectiveness of the coronavirus disease 2019 bivalent vaccine. Open Forum Infect Dis. 2023; 10(6):ofad209. PMID: 37274183.13. Korea Disease Control and Prevention Agency. Public Health Weekly Report for COVID-19, February 1, 2023. Cheongju, Korea: Korea Disease Control and Prevention Agency;2023.14. Qu P, Evans JP, Faraone JN, Zheng YM, Carlin C, Anghelina M, et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe. 2023; 31(1):9–17.e3. PMID: 36476380.15. Cao Y, Jian F, Wang J, Yu Y, Song W, Yisimayi A, et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2023; 614(7948):521–529. PMID: 36535326.16. Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults - increasing community access to testing program, United States, December 2022–January 2023. MMWR Morb Mortal Wkly Rep. 2023; 72(5):119–124. PMID: 36730051.17. Link-Gelles R, Weber ZA, Reese SE, Payne AB, Gaglani M, Adams K, et al. Estimates of bivalent mRNA vaccine durability in preventing COVID-19-associated hospitalization and critical illness among adults with and without immunocompromising conditions - vision network, September 2022–April 2023. MMWR Morb Mortal Wkly Rep. 2023; 72(21):579–588. PMID: 37227984.18. Xia H, Zou J, Kurhade C, Cai H, Yang Q, Cutler M, et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe. 2022; 30(4):485–488.e3. PMID: 35245438.19. Kurhade C, Zou J, Xia H, Liu M, Chang HC, Ren P, et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat Med. 2023; 29(2):344–347. PMID: 36473500.20. Fabiani M, Mateo-Urdiales A, Sacco C, Fotakis EA, Rota MC, Petrone D, et al. Protection against severe COVID-19 after second booster dose of adapted bivalent (original/Omicron BA.4-5) mRNA vaccine in persons ≥60 years, by time since infection, Italy, 12 September to 11 December 2022. Euro Surveill. 2023; 28(8):2300105. PMID: 36820640.21. Kirsebom FCM, Andrews N, Stowe J, Ramsay M, Lopez Bernal J. Duration of protection of ancestral-strain monovalent vaccines and effectiveness of bivalent BA.1 boosters against COVID-19 hospitalisation in England: a test-negative case-control study. Lancet Infect Dis. 2023; 23(11):1235–1243. PMID: 37453440.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effectiveness of the Bivalent mRNA COVID-19 Vaccine for Preventing Critical Infection From the SARS-CoV-2 Omicron Variant in the Republic of Korea
- Comparative Effectiveness of COVID-19 Bivalent Versus Monovalent mRNA Vaccines in the Early Stage of Bivalent Vaccination in Korea: October 2022 to January 2023
- Comparative safety of monovalent and bivalent mRNA COVID-19 booster vaccines in adolescents aged 12 to 17 years in the Republic of Korea
- COVID-19 Vaccination in Korea
- Low Neutralizing Activities to the Omicron Subvariants BN.1 and XBB.1.5 of Sera From the Individuals Vaccinated With a BA.4/5-Containing Bivalent mRNA Vaccine