Ann Clin Microbiol.
2023 Dec;26(4):125-137. 10.5145/ACM.2023.26.4.125.
Performance evaluation of 4-day versus 5-day blood cultures using the BD BACTEC FX system
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2550664
- DOI: http://doi.org/10.5145/ACM.2023.26.4.125
Abstract
- Background
Blood culture (BC) systems have evolved to increase sensitivity and reduce turnaround times. This study compared the performance of a 4-day versus a 5-day BC incubation period using the BD BACTEC™ FX (Becton, Dickinson and Company, USA).
Methods
A total of 37,379 consecutive sets of BC were evaluated over a 3-month period in a 2,700-bed tertiary care hospital. Positive BC results were reviewed to assess time-to-positivity (TTP) and species identification of the isolates. The BCs were performed in pairs of vials, utilizing either BD BACTEC Plus Aerobic/F or Peds Plus/F with BD BACTEC Lytic Anaerobic media.
Results
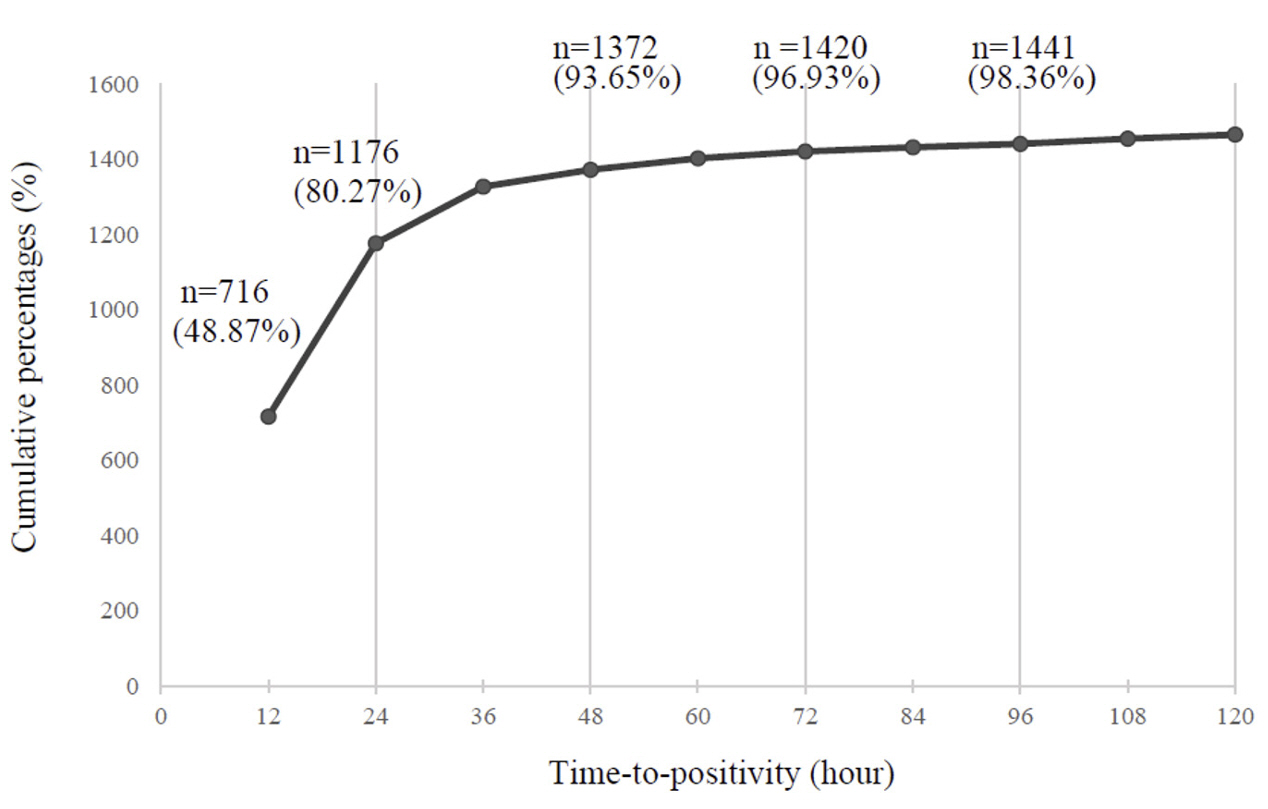
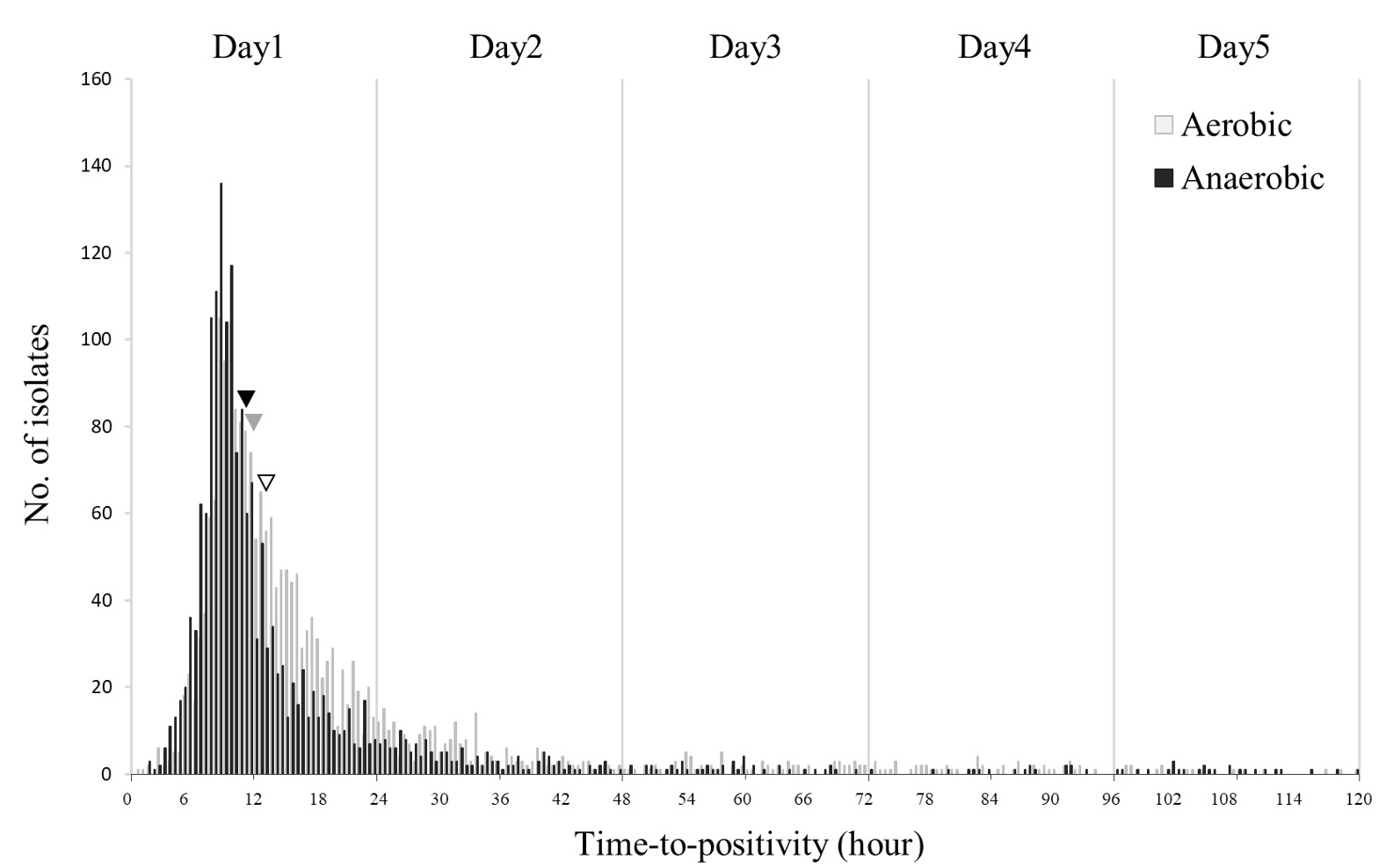
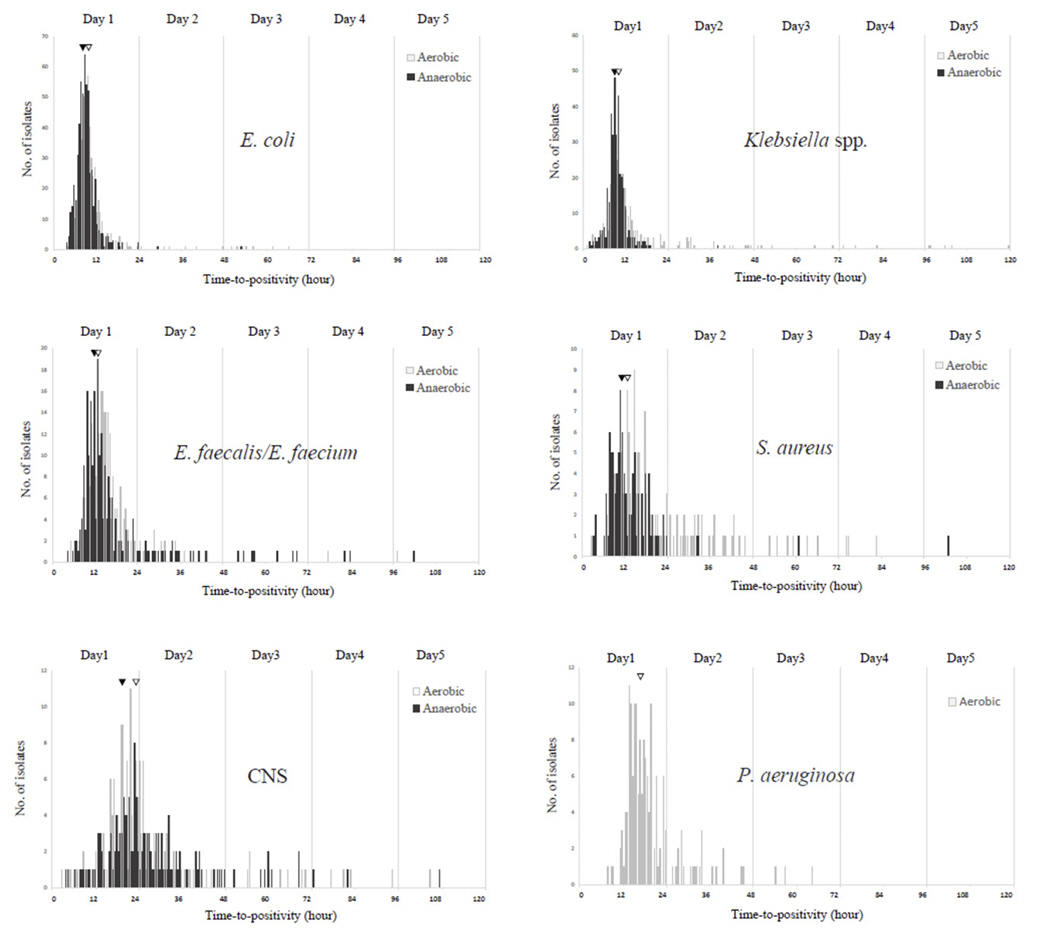
A total of 14,899 episodes, averaging 2.51 sets per episode, were analyzed. Of these, 1,398 (9.38%) were positive, yielding 1,465 isolates. TTP (hours) were < 12 in 48.87%, 12-24 in 31.40%, 24-48 in 13.38%, 48-72 in 3.28%, 72-96 in 1.43%, and >96 in 1.64%. The two most prevalent organisms, Escherichia coli and Klebsiella spp. were detected within 12 hours in 88.75% and 78.90%, respectively. The respective median TTP (T50) values for E. coli, Klebsiella spp., Enterococcus faecalis/E. faecium, and Staphylococcus aureus were 9.24, 9.60, 13.75, and 14.20. T50 values for these species were significantly shorter in anaerobic bottles than in aerobic bottles. Of 24 BCs with TTP > 96, only 4 containing anaerobic bacteria or molds were first detected after 96 hours.
Conclusion
A 4-day incubation has demonstrated excellent sensitivity. However, a 5-day incubation may be beneficial for hospitals caring for patients at high risk for infections with slow-growing fungi or fastidious bacteria.
Figure
Reference
-
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013;19:501-9. .2. Pien BC, Sundaram P, Raoof N, Costa SF, Mirrett S, Woods CW, et al. The clinical and prognostic importance of positive blood cultures in adults. Am J Med 2010;123:819-28. .3. Hardy DJ, Hulbert BB, Migneault PC. Time to detection of positive BacT/Alert blood cultures and lack of need for routine subculture of 5- to 7-day negative cultures. J Clin Microbiol 1992;30:2743-5. .4. Seo EJ and Pai CH. BACTEC NR-730 blood culture. Korean J Clin Pathol 1993;13:601-6. .5. Clinical and Laboratory Standards Institute. Principles and procedures for blood cultures. 2nd ed. CLSI guideline M47. Wayne, PA: CLSI; 2022. .6. Clinical and Laboratory Standards Institute. Principle and procedures for blood cultures; approved guideline. CLSI document M47-A. Wayne, PA: CLSI; 2007. .7. Sung H, Kim MN, Pai CH. The clinical relevance of four-day blood cultures with the BACTEC 9240 system. Korean J Clin Pathol 2001:193-8. .8. Ransom EM, Alipour Z, Wallace MA, Burnham CA. Evaluation of optimal blood culture incubation time to maximize clinically relevant results from a contemporary blood culture instrument and media system. J Clin Microbiol 2021;59:e02459-20. .9. Doern GV, Brueggemann AB, Dunne WM, Jenkins SG, Halstead DC, McLaughlin JC. Fourday incubation period for blood culture bottles processed with the Difco ESP blood culture system. J Clin Microbiol 1997;35:1290-2. .10. Bourbeau PP and Pohlman JK. Three days of incubation may be sufficient for routine blood cultures with BacT/Alert FAN blood culture bottles. J Clin Microbiol 2001;39:2079-82. .11. Park SH, Shim H, Yoon NS, Kim MN. Clinical relevance of time-to-positivity in BACTEC9240 blood culture system. Korean J Lab Med 2010;30:276-83. .12. Pardo J, Klinker KP, Borgert SJ, Trikha G, Rand KH, Ramphal R. Time to positivity of blood cultures supports antibiotic de-escalation at 48 hours. Ann Pharmacother 2014;48:33-40. .13. Barenfanger J, Graham DR, Kolluri L, Sangwan G, Lawhorn J, Drake CA, et al. Decreased mortality associated with prompt Gram staining of blood cultures. Am J Clin Pathol 2008;130:870-6. .14. López-Pintor JM, Sánchez-López J, Navarro-San Francisco C, Sánchez-Díaz AM, Loza E, Cantón R. Real life clinical impact of antimicrobial stewardship actions on the blood culture workflow from a microbiology laboratory. Antibiotics 2021;10:1511. .15. Park HJ, Na S, Park SY, Moon SM, Cho OH, Park KH, et al. Clinical significance of Propionibacterium acnes recovered from blood cultures: analysis of 524 episodes. J Clin Microbiol 2011;49:1598-601. .16. Tusgul S, Prod'hom G, Senn L, Meuli R, Bochud PY, Giulieri SG. Bacillus cereus bacteraemia: comparison between haematologic and nonhaematologic patients. New Microbes New Infect 2017;15:65-71. .17. Song SA, Kim JH, Shin JH, Kim SH, Lee NY, Kim MN, et al. Clinical usefulness of routine use of anaerobic blood culture bottle. Ann Clin Microbiol 2014;17:35-41. .18. Ransom EM and Burnham CA. Routine use of anaerobic blood culture bottles for specimens collected from adults and children enhances microorganism recovery and improves time to positivity. J Clin Microbiol 2022;60:e00500-22. .19. Viganò EF, Vasconi E, Agrappi C, Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT™ and BACTEC™ 9240 blood culture systems. Diagn Microbiol Infect Dis 2002;44:235-40. .20. Cobos-Trigueros N, Zboromyrska Y, Morata L, Alejo-Cancho I, Calle CD, Vergara Gómez A, et al. Time-to-positivity, type of culture media and oxidase test performed on positive blood culture vials to predict Pseudomonas aeruginosa in patients with Gram-negative bacilli bacteraemia. Rev Esp Quimioter 2017;30:9-13. .21. Klaerner HG, Eschenbach U, Kamereck K, Lehn N, Wagner H, Miethke T. Failure of an automated blood culture system to detect nonfermentative gram-negative bacteria. J Clin Microbiol 2000;38:1036-41. .22. Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 2006;50:43-8. .23. Lee JY, Hong JH, Lee M. Comparison of BACTEC Plus aerobic/F media and BacT/Alert FA media to detect bacteria in blood culture bottles containing peak therapeutic levels of antimicrobials. Korean J Clin Microbiol 2010;13:151-6. .24. Doern GV. Detection of selected fastidious bacteria. Clin Infect Dis 2000;30:166-73. .25. Reisner BS and Woods GL. Times to detection of bacteria and yeasts in BACTEC 9240 blood culture bottles. J Clin Microbiol 1999;37:2024-6. .26. Baron EJ, Scott JD, Tompkins LS. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures. Clin Infect Dis 2005;41:1677-80. .27. Petti CA, Bhally HS, Weinstein MP, Joho K, Wakefield T, Reller LB, et al. Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella organisms: a retrospective multicenter evaluation. J Clin Microbiol 2006;44:257-9. .28. Weinstein MP. Emerging data indicating that extended incubation of blood cultures has little clinical value. Clin Infect Dis 2005;41:1681-2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Relevance of Four-Day Blood Cultures with the BACTEC 9240 System
- Comparison of the BACTEC Peds Plus Pediatric Blood Culture Bottle to the BacT/Alert PF Pediatric Blood Culture Bottle for Culturing Blood from Pediatric Patients
- Detection of Mycobacterium tuberculosis using BACTEC Mycobacteria Growth Indicator Tube(MGIT) 960 system: Comparison with BACTEC 460 TB system and Ogawa Media
- Evaluation of BACTEC PLUS Resin Media with The BACTEC 9240 System
- A Case of Brain Abscess Caused by Propionibacterium acnes 13 Months after Neurosurgery and Confirmed by 16S rRNA Gene Sequencing