Cancer Res Treat.
2024 Jan;56(1):191-198. 10.4143/crt.2022.958.
Functional Annotation and Gene Set Analysis of Gastric Cancer Risk Loci in a Korean Population
- Affiliations
-
- 1Division of Genome and Health Big Data, Department of Public Health Sciences, Graduate School of Public Health, Seoul National University, Seoul, Korea
- 2Department of Cancer Biomedical Science, Graduate School of Cancer Science and Policy, Goyang, Korea
- 3Center for Gastric Cancer, National Cancer Center Hospital, National Cancer Center, Goyang, Korea
- 4Institute of Health and Environment, Seoul National University, Seoul, Korea
- KMID: 2550335
- DOI: http://doi.org/10.4143/crt.2022.958
Abstract
- Purpose
We aimed to identify the associated single nucleotide polymorphisms (SNPs) with gastric cancer (GC) risk by genome-wide association study (GWAS) and to explore the pathway enrichment of implicated genes and gene-sets with expression patterns.
Materials and Methods
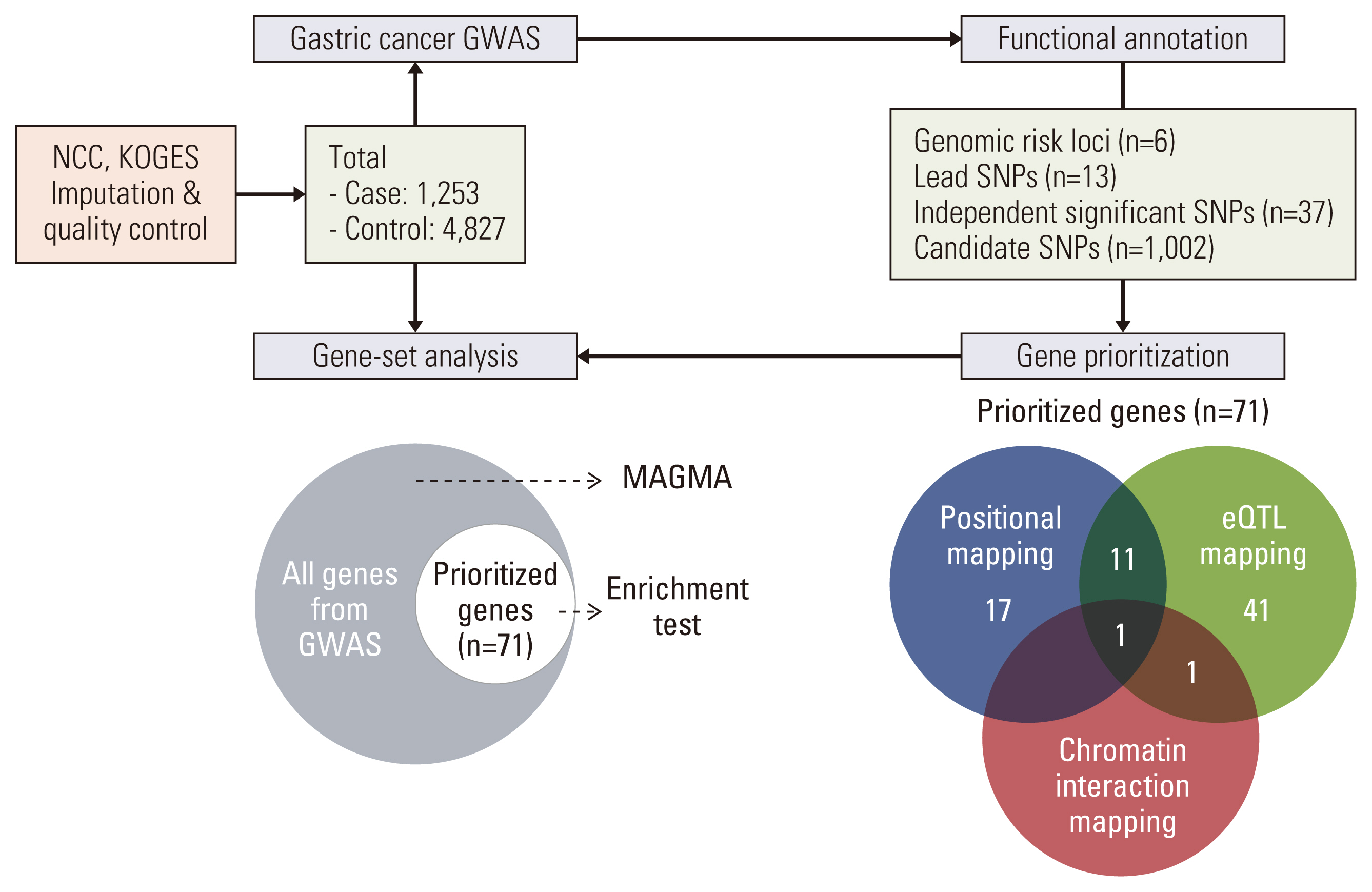
The study population was comprised of 1,253 GC cases and 4,827 controls from National Cancer Center and an urban community of the Korean Genome Epidemiology Study and their genotyping was performed. SNPs were annotated, and mapped to genes to prioritize by three mapping approaches by functional mapping and annotation (FUMA). The gene-based analysis and gene-set analysis were conducted with full GWAS summary data using MAGMA. Gene-set pathway enrichment test with those prioritized genes were performed.
Results
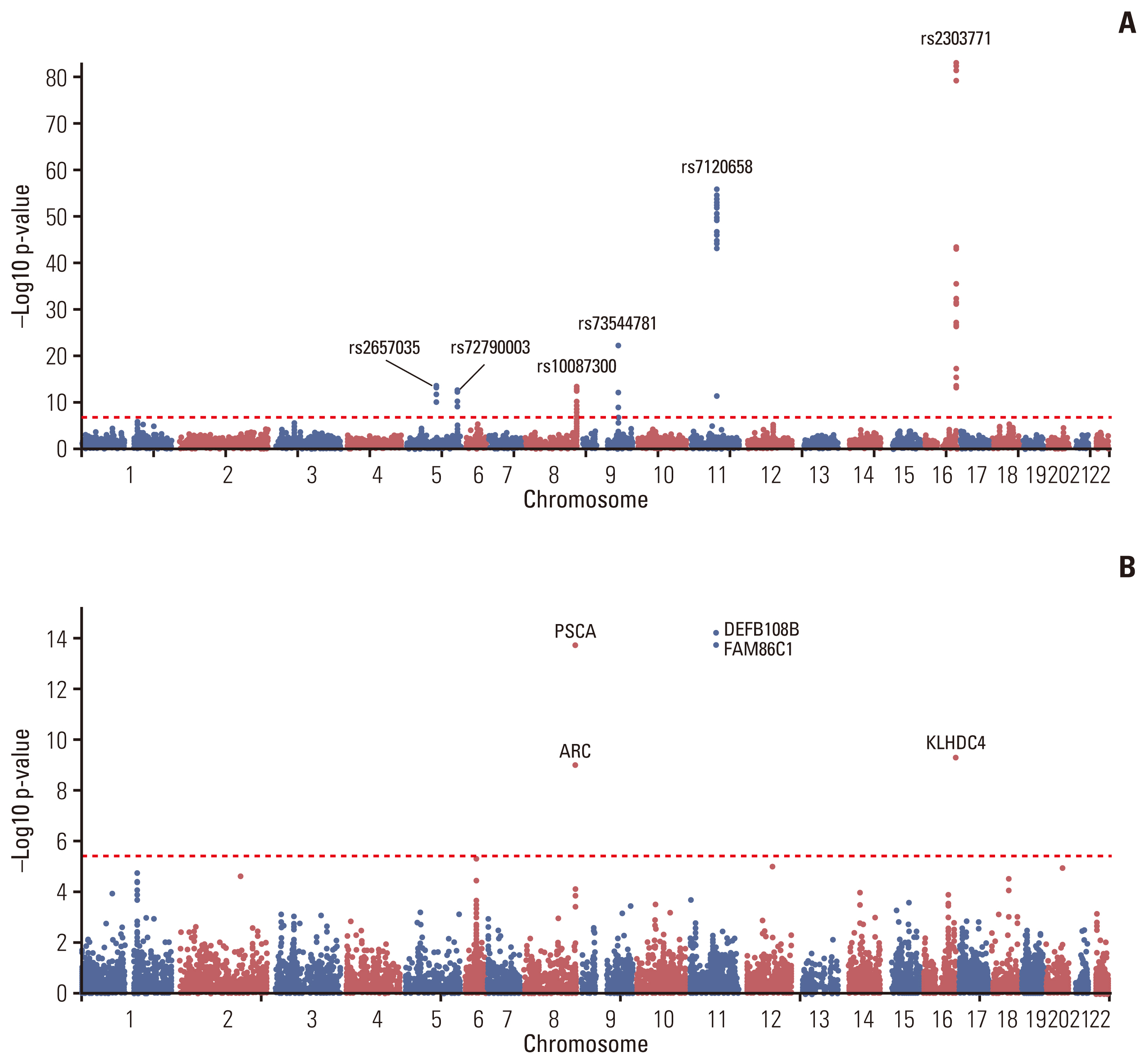
In GWAS, rs2303771, a nonsynonymous variant of KLHDC4 gene was top SNP associated significantly with GC (odds ratio, 2.59; p=1.32×10–83). In post-GWAS, 71 genes were prioritized. In gene-based GWAS, seven genes were under significant p < 3.80×10–6 (0.05/13,114); DEFB108B had the lowest p=5.94×10–15, followed by FAM86C1 (p=1.74×10–14), PSCA (p=1.81×10–14), and KLHDC4 (p=5.00×10–10). In gene prioritizing, KLDHC4 was the only gene mapped with all three gene-mapping approaches. In pathway enrichment test with prioritized genes, FOLR2, PSCA, LY6K, LYPD2, and LY6E showed strong enrichment related to cellular component of membrane; a post-translation modification by synthesis of glycosylphosphatidylinositol (GPI)-anchored proteins pathway.
Conclusion
While 37 SNPs were significantly associated with the risk of GC, genes involved in signaling pathways related to purine metabolism and GPI-anchored protein in cell membrane are pinpointed to be playing important role in GC.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.2. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021; 53:301–15.3. Akbari A, Ashtari S, Tabaiean SP, Mehrdad-Majd H, Farsi F, Shojaee S, et al. Overview of epidemiological characteristics, clinical features, and risk factors of gastric cancer in Asia-Pacific region. Asia Pac J Clin Oncol. 2022; 18:493–505.4. Thrastardottir TO, Copeland VJ, Constantinou C. The association between the gut microbiome, nutritional habits, antibiotics, and gastric cancer: a scoping review. Curr Nutr Rep. 2022; 11:19–38.5. McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014; 11:664–74.6. Study Group of Millennium Genome Project for Cancer, Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008; 40:730–40.7. Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010; 42:764–7.8. Shi Y, Hu Z, Wu C, Dai J, Li H, Dong J, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat Genet. 2011; 43:1215–8.9. Jin G, Ma H, Wu C, Dai J, Zhang R, Shi Y, et al. Genetic variants at 6p21.1 and 7p15.3 are associated with risk of multiple cancers in Han Chinese. Am J Hum Genet. 2012; 91:928–34.10. Zhao Y, Liang X, Zhu F, Wen Y, Xu J, Yang J, et al. A large-scale integrative analysis of GWAS and common meQTLs across whole life course identifies genes, pathways and tissue/cell types for three major psychiatric disorders. Neurosci Biobehav Rev. 2018; 95:347–52.11. Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016; 48:481–7.12. Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014; 42:D1001–6.13. Gallagher MD, Chen-Plotkin AS. The post-GWAS era: from association to function. Am J Hum Genet. 2018; 102:717–30.14. Freedman ML, Monteiro AN, Gayther SA, Coetzee GA, Risch A, Plass C, et al. Principles for the post-GWAS functional characterization of cancer risk loci. Nat Genet. 2011; 43:513–8.15. Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc. 2019; 14:482–517.16. Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017; 8:1826.17. 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015; 526:68–74.18. Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014; 46:310–5.19. Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012; 22:1790–7.20. GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020; 369:1318–30.21. Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods. 2012; 9:215–6.22. The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489:57–74.23. Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015; 518:317–30.24. Schmiedel BJ, Singh D, Madrigal A, Valdovino-Gonzalez AG, White BM, Zapardiel-Gonzalo J, et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell. 2018; 175:1701–15.25. Vosa U, Claringbould A, Westra HJ, Bonder MJ, Deelen P, Zeng B, et al. Unraveling the polygenic architecture of complex traits using blood eQTL metaanalysis. Preprint at https://www.biorxiv.org/content/10.1101/447367v1 (2018).26. de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015; 11:e1004219.27. Park B, Yang S, Lee J, Woo HD, Choi IJ, Kim YW, et al. Genome-wide association of genetic variation in the PSCA gene with gastric cancer susceptibility in a Korean population. Cancer Res Treat. 2019; 51:748–57.28. Kim YD, Yim DH, Eom SY, Moon SI, Yun HY, Song YJ, et al. Risk of gastric cancer is associated with PRKAA1 gene polymorphisms in Koreans. World J Gastroenterol. 2014; 20:8592–8.29. Song HR, Kim HN, Kweon SS, Choi JS, Shim HJ, Cho SH, et al. Common genetic variants at 1q22 and 10q23 and gastric cancer susceptibility in a Korean population. Tumour Biol. 2014; 35:3133–7.30. Hwang JY, Kim DH, Ji YI, Go MJ, Heo L, Kim YJ, et al. Recapitulation of previous genome-wide association studies with two distinct pathophysiological entities of gastric cancer in the Korean population. J Hum Genet. 2013; 58:233–5.31. Cai M, Dai S, Chen W, Xia C, Lu L, Dai S, et al. Environmental factors, seven GWAS-identified susceptibility loci, and risk of gastric cancer and its precursors in a Chinese population. Cancer Med. 2017; 6:708–20.32. Qiu L, Qu X, He J, Cheng L, Zhang R, Sun M, et al. Predictive model for risk of gastric cancer using genetic variants from genome-wide association studies and high-evidence meta-analysis. Cancer Med. 2020; 9:7310–6.33. Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004; 5:110–20.34. Lv Y, Song Y, Ni C, Wang S, Chen Z, Shi X, et al. Overexpression of lymphocyte antigen 6 complex, locus E in gastric cancer promotes cancer cell growth and metastasis. Cell Physiol Biochem. 2018; 45:1219–29.35. Yeom CJ, Zeng L, Goto Y, Morinibu A, Zhu Y, Shinomiya K, et al. LY6E: a conductor of malignant tumor growth through modulation of the PTEN/PI3K/Akt/HIF-1 axis. Oncotarget. 2016; 7:65837–48.36. Li E, Liu L, Li F, Luo L, Zhao S, Wang J, et al. PSCA promotes prostate cancer proliferation and cell-cycle progression by up-regulating c-Myc. Prostate. 2017; 77:1563–72.37. Craig SE, Brady-Kalnay SM. Cancer cells cut homophilic cell adhesion molecules and run. Cancer Res. 2011; 71:303–9.38. Niu XM, Lu S. Acetylcholine receptor pathway in lung cancer: New twists to an old story. World J Clin Oncol. 2014; 5:667–76.39. Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, et al. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G591–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- GSnet: An Integrated Tool for Gene Set Analysis and Visualization
- SFannotation: A Simple and Fast Protein Function Annotation System
- The Metformin Use and Gastric Cancer Risk
- Loss of heterozygosity at the MCC and APC genetic loci in precancerous gastric lesion and gastric cancer
- Subepithelial Tumor-like Gastric Cancer