Ann Surg Treat Res.
2024 Jan;106(1):19-30. 10.4174/astr.2024.106.1.19.
Effect of thyroid-stimulating hormone suppression on quality of life in thyroid lobectomy patients: interim analysis of a multicenter, randomized controlled trial in low- to intermediate-risk thyroid cancer patients (MASTER study)
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea
- 3Department of Surgery, Seoul National University Hospital, Seoul, Korea
- 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 5Cancer Research Institute, Seoul National University, Seoul, Korea
- 6Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Nowon Eulji Medical Center, Seoul, Korea
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea
- 9Department of Internal Medicine, Pusan National University School of Medicine, Yangsan, Korea
- 10Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 11Department of Internal Medicine, Chonnam National University Hwasun Hospital and Medical School, Hwasun, Korea
- 12Department of Otolaryngology-Head and Neck Surgery, Center for Thyroid Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- KMID: 2550247
- DOI: http://doi.org/10.4174/astr.2024.106.1.19
Abstract
- Purpose
Current clinical practices favor less or no thyroid-stimulating hormone (TSH) suppression for low- to intermediate-risk thyroid cancer patients who receive thyroid lobectomy. The association of TSH suppression on healthrelated quality of life (HR-QoL) in patients after thyroid lobectomy is not well studied. This study aimed to evaluate the effect of TSH suppression on patient HR-QoL after thyroid lobectomy.
Methods
This study included patients enrolled in an ongoing, multicenter, randomized controlled study investigating the effects of TSH suppression. Patients were randomized to either the low-TSH group (TSH target range, 0.3–1.99 μIU/ mL) or the high-TSH group (TSH target range, 2.0–7.99 μIU/mL). The HR-QoL, hyperthyroidism symptom, and depression symptom questionnaires performed preoperatively and 2 weeks and 3 months postoperatively were evaluated.
Results
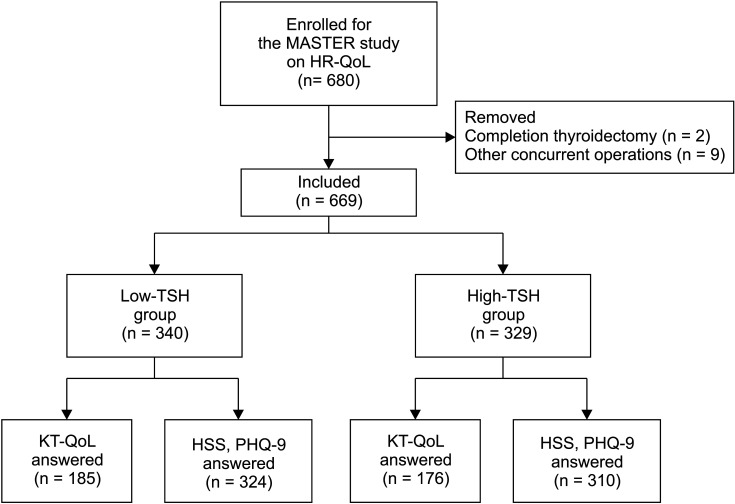
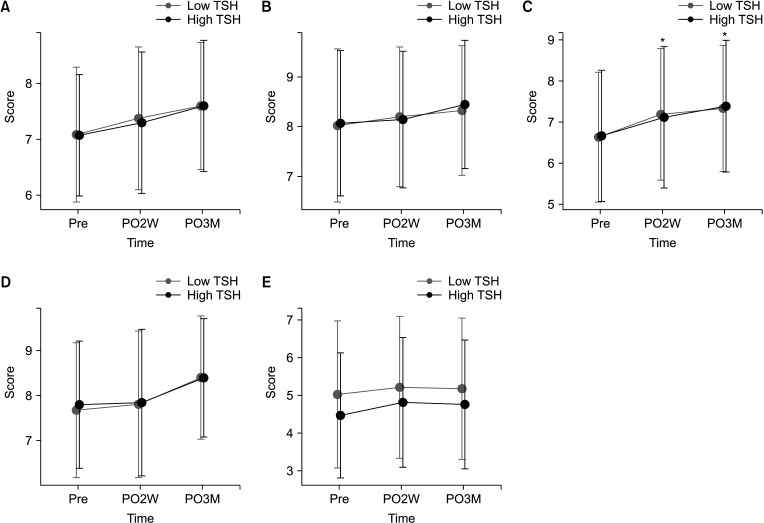
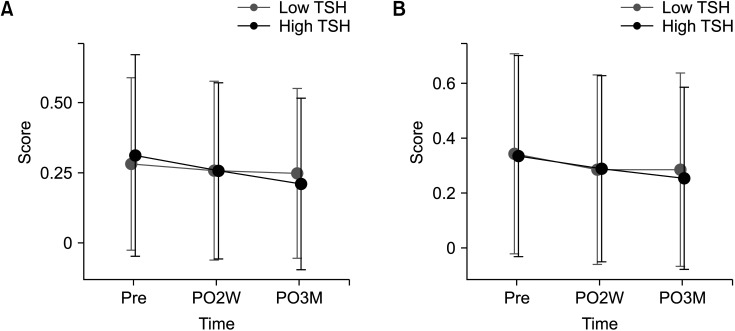
Total of 669 patients (low-TSH group, 340; high-TSH group, 329) were included. Although total HR-QoL score changes were not different between the 2 groups, the high-TSH group had a significantly higher score in the physical domain at postoperative 3 months (P = 0.046). The 2 groups did not have significant differences in hyperthyroidism and depression scores.
Conclusion
In the short-term postoperative period, the physical HR-QoL scores in thyroid lobectomy patients were better when they did not receive TSH suppression. This study suggests the importance of considering HR-QoL when setting TSH suppression targets in thyroid lobectomy patients.
Figure
Cited by 2 articles
-
Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part II. Follow-up Surveillance after Initial Treatment 2024
Mijin Kim, Ji-In Bang, Ho-Cheol Kang, Sun Wook Kim, Dong Gyu Na, Young Joo Park, Youngduk Seo, Young Shin Song, So Won Oh, Sang-Woo Lee, Eun Kyung Lee, Ji Ye Lee, Dong-Jun Lim, Ari Chong, Yun Jae Chung, Chae Moon Hong, Min Kyoung Lee, Bo Hyun Kim
Int J Thyroidol. 2024;17(1):115-146. doi: 10.11106/ijt.2024.17.1.115.Levothyroxine Dosing for Thyroid-Stimulating Hormone Suppression in Patients with Differentiated Thyroid Cancer after Total Thyroidectomy
Mijin Kim
Endocrinol Metab. 2024;39(4):576-578. doi: 10.3803/EnM.2024.401.
Reference
-
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.
Article2. McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013; 381:1046–1057. PMID: 23668555.
Article3. Patel KN. The year in thyroidology: surgical science. Thyroid. 2023; 33:21–23. PMID: 36511381.
Article4. Lee MC, Kim MJ, Choi HS, Cho SW, Lee GH, Park YJ, et al. Postoperative thyroid-stimulating hormone levels did not affect recurrence after thyroid lobectomy in patients with papillary thyroid cancer. Endocrinol Metab (Seoul). 2019; 34:150–157. PMID: 31099202.5. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006; 16:1229–1242. PMID: 17199433.6. Carhill AA, Litofsky DR, Ross DS, Jonklaas J, Cooper DS, Brierley JD, et al. Long-Term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS Registry analysis 1987-2012. J Clin Endocrinol Metab. 2015; 100:3270–3279. PMID: 26171797.7. Park S, Kim WG, Han M, Jeon MJ, Kwon H, Kim M, et al. Thyrotropin suppressive therapy for low-risk small thyroid cancer: a propensity score-matched cohort study. Thyroid. 2017; 27:1164–1170. PMID: 28699428.8. Tagay S, Herpertz S, Langkafel M, Erim Y, Freudenberg L, Schöpper N, et al. Health-related quality of life, anxiety and depression in thyroid cancer patients under short-term hypothyroidism and TSH-suppressive levothyroxine treatment. Eur J Endocrinol. 2005; 153:755–763. PMID: 16322380.9. Grani G, Ramundo V, Verrienti A, Sponziello M, Durante C. Thyroid hormone therapy in differentiated thyroid cancer. Endocrine. 2019; 66:43–50. PMID: 31617165.10. Yaniv D, Vainer I, Amir I, Robenshtok E, Hirsch D, Watt T, et al. Quality of life following lobectomy versus total thyroidectomy is significantly related to hypothyroidism. J Surg Oncol. 2022; 126:640–648. PMID: 35689620.11. Monzani ML, Piccinini F, Boselli G, Corleto R, Margiotta G, Peeters RP, et al. Changes in quality of life after thyroidectomy in subjects with thyroid cancer in relation to the dose of levothyroxine. J Endocrinol Invest. 2023; 46:319–326. PMID: 35988109.12. Lee EK, Kang YE, Park YJ, Koo BS, Chung KW, Ku EJ, et al. A multicenter, randomized, controlled trial for assessing the usefulness of suppressing thyroid stimulating hormone target levels after thyroid lobectomy in low to intermediate risk thyroid cancer patients (MASTER): a study protocol. Endocrinol Metab (Seoul). 2021; 36:574–581. PMID: 34034365.13. Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997; 7:613–619. PMID: 9292951.14. Ryu CH, Park B, Ryu J, Ryu YM, Jo SA, Lee YJ, et al. Development and evaluation of a Korean version of a Thyroid-Specific Quality-of-Life Questionnaire Scale in thyroid cancer patients. Cancer Res Treat. 2018; 50:405–415. PMID: 28602058.15. Klein I, Trzepacz PT, Roberts M, Levey GS. Symptom rating scale for assessing hyperthyroidism. Arch Intern Med. 1988; 148:387–390. PMID: 3124776.16. Lee JE, Lee DH, Oh TJ, Kim KM, Choi SH, Lim S, et al. Validity and reliability of the Korean version of the Hyperthyroidism Symptom Scale. Endocrinol Metab (Seoul). 2018; 33:70–78. PMID: 29589389.17. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16:606–613. PMID: 11556941.18. Park SJ, Choi HR, Choi JH, Kim KW, Hong JP. Reliability and validity of the Korean version of the Patient Health Questionnaire-9 (PHQ-9). Anxiety Mood. 2010; 6:119–124.19. Goldfarb M, Casillas J. Thyroid cancer-specific quality of life and health-related quality of life in young adult thyroid cancer survivors. Thyroid. 2016; 26:923–932. PMID: 27161396.20. Chen W, Li J, Peng S, Hong S, Xu H, Lin B, et al. Association of total thyroidectomy or thyroid lobectomy with the quality of life in patients with differentiated thyroid cancer with low to intermediate risk of recurrence. JAMA Surg. 2022; 157:200–209. PMID: 34935859.21. Moon JH, Ryu CH, Cho SW, Choi JY, Chung EJ, Hah JH, et al. Effect of initial treatment choice on 2-year quality of life in patients with low-risk papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2021; 106:724–735. PMID: 33248442.22. Dou Y, Chen Y, Hu D, Su X. The recovery of thyroid function in low-risk papillary thyroid cancer after lobectomy: a 3-year follow-up study. Front Endocrinol (Lausanne). 2021; 11:619841. PMID: 33633689.23. Bae MR, Nam SH, Roh JL, Choi SH, Nam SY, Kim SY. Thyroid stimulating hormone suppression and recurrence after thyroid lobectomy for papillary thyroid carcinoma. Endocrine. 2022; 75:487–494. PMID: 34689317.24. Sheehan MT. Biochemical testing of the thyroid: TSH is the best and, oftentimes, only test needed: a review for primary care. Clin Med Res. 2016; 14:83–92. PMID: 27231117.25. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003; 115:151–162. PMID: 14567913.26. Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab. 2018; 103:1997–2008. PMID: 29509918.27. Kim BJ, Lee SH, Isales CM, Hamrick MW, Kwak MK, Koh JM. Association of serum TSH with handgrip strength in community-dwelling euthyroid elderly. J Clin Endocrinol Metab. 2018; 103:3986–3992. PMID: 30137405.28. Moon MK, Kang GH, Kim HH, Han SK, Koo YD, Cho SW, et al. Thyroid-stimulating hormone improves insulin sensitivity in skeletal muscle cells via cAMP/PKA/CREB pathway-dependent upregulation of insulin receptor substrate-1 expression. Mol Cell Endocrinol. 2016; 436:50–58. PMID: 27452800.29. Deng T, Zhang W, Zhang Y, Zhang M, Huan Z, Yu C, et al. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr Disord. 2021; 21:49. PMID: 33726721.30. Crevenna R, Zettinig G, Keilani M, Posch M, Schmidinger M, Pirich C, et al. Quality of life in patients with non-metastatic differentiated thyroid cancer under thyroxine supplementation therapy. Support Care Cancer. 2003; 11:597–603. PMID: 12783288.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TSH Suppression after Differentiated Thyroid Cancer Surgery and Osteoporosis
- Postoperative Thyroid-Stimulating Hormone Levels Did Not Affect Recurrence after Thyroid Lobectomy in Patients with Papillary Thyroid Cancer
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: a Position Statement from the Korean Thyroid Association
- Risk Factors for Hypothyroidism after Thyroid Lobectomy with Papillary Thyroid Crcinoma according to Existence of Thyroiditis
- Clinical Factors Associated with Quality of Life in Patients with Thyroid Cancer