Clin Exp Otorhinolaryngol.
2023 Nov;16(4):326-333. 10.21053/ceo.2022.00423.
Increased Resting-State Positron Emission Tomography Activity After Cochlear Implantation in Adult Deafened Cats
- Affiliations
-
- 1Department of Otorhinolaryngology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Otorhinolaryngology, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 3Division of RI Application, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 4Sensory Organ Research Institute, Seoul National University Medical Research Center, Seoul, Korea
- KMID: 2548361
- DOI: http://doi.org/10.21053/ceo.2022.00423
Abstract
Objectives
. Cochlear implants are widely used for hearing rehabilitation in patients with profound sensorineural hearing loss. However, Cochlear implants have variable results, and central neural plasticity is considered to be a reason for this variability. We hypothesized that resting-state cortical networks play a role in conditions of profound hearing loss and are affected by cochlear implants. To investigate the resting-state neuronal networks after cochlear implantation, we acquired 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) images in experimental animals.
Methods
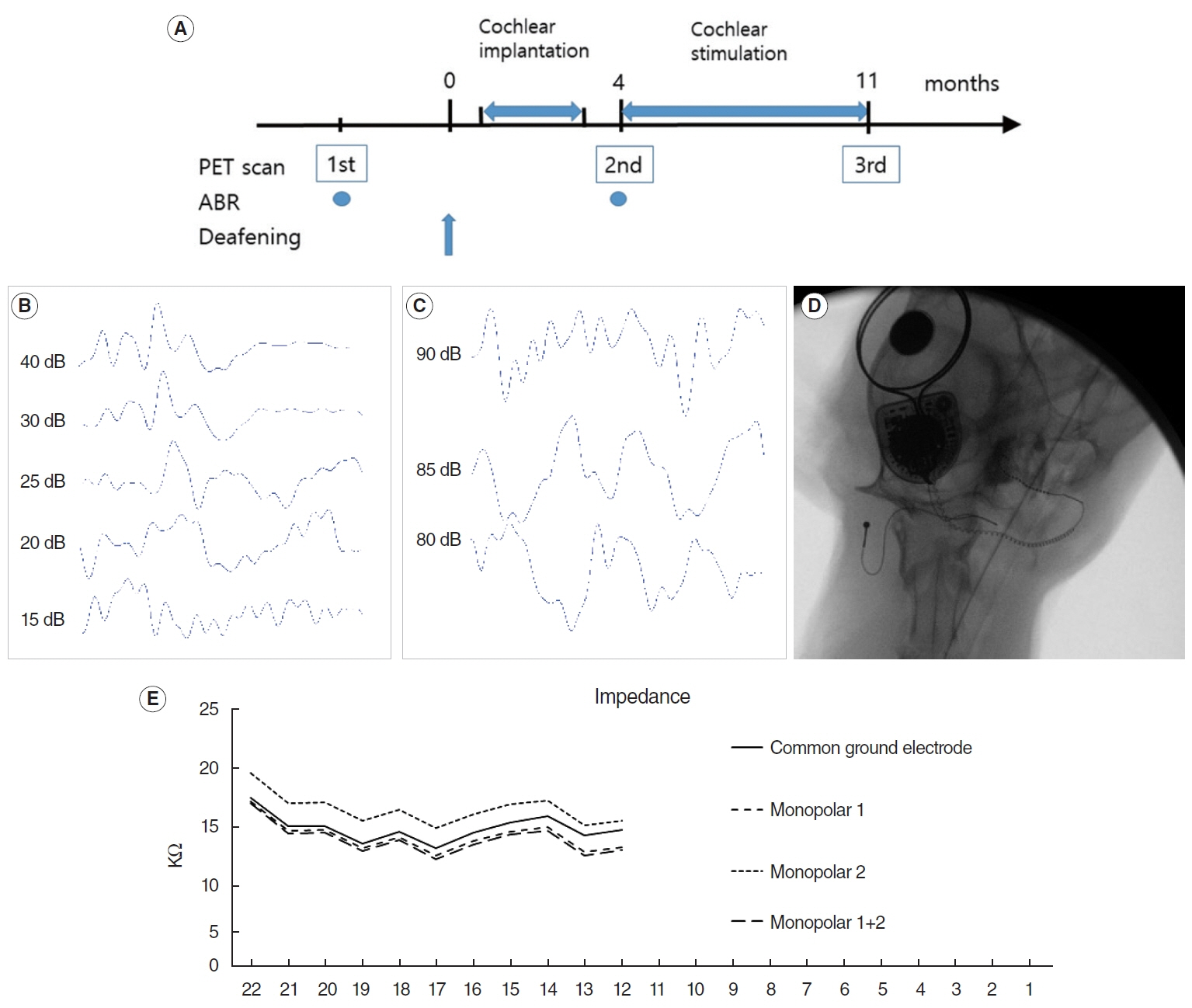
. Eight adult domestic cats were enrolled in this study. The hearing threshold of the animals was within the normal range, as measured by auditory evoked potential. They were divided into control (n=4) and hearing loss (n=4) groups. Hearing loss was induced by co-administration of ethacrynic acid and kanamycin. FDG-PET was performed in a normal hearing state and 4 and 11 months after the deafening procedure. Cochlear implantation was performed in the right ear, and electrical cochlear stimulation was performed for 7 months (from 4 to 11 months after the deafening procedure). PET images were compared between the two groups at the three time points.
Results
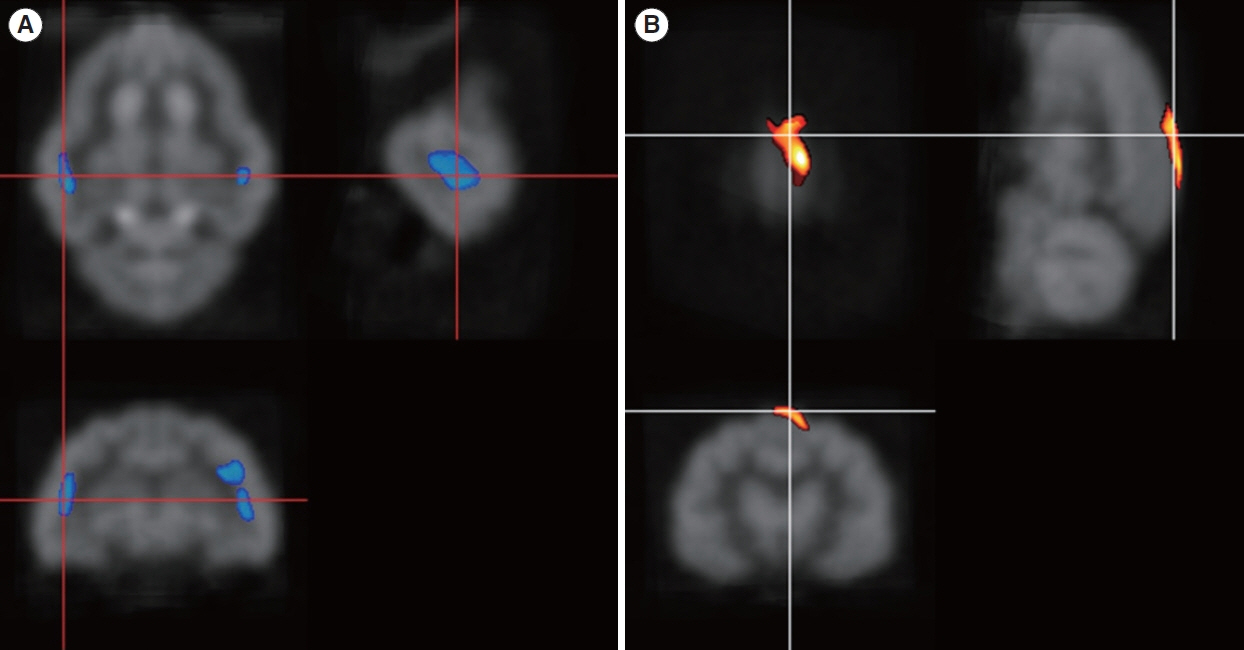
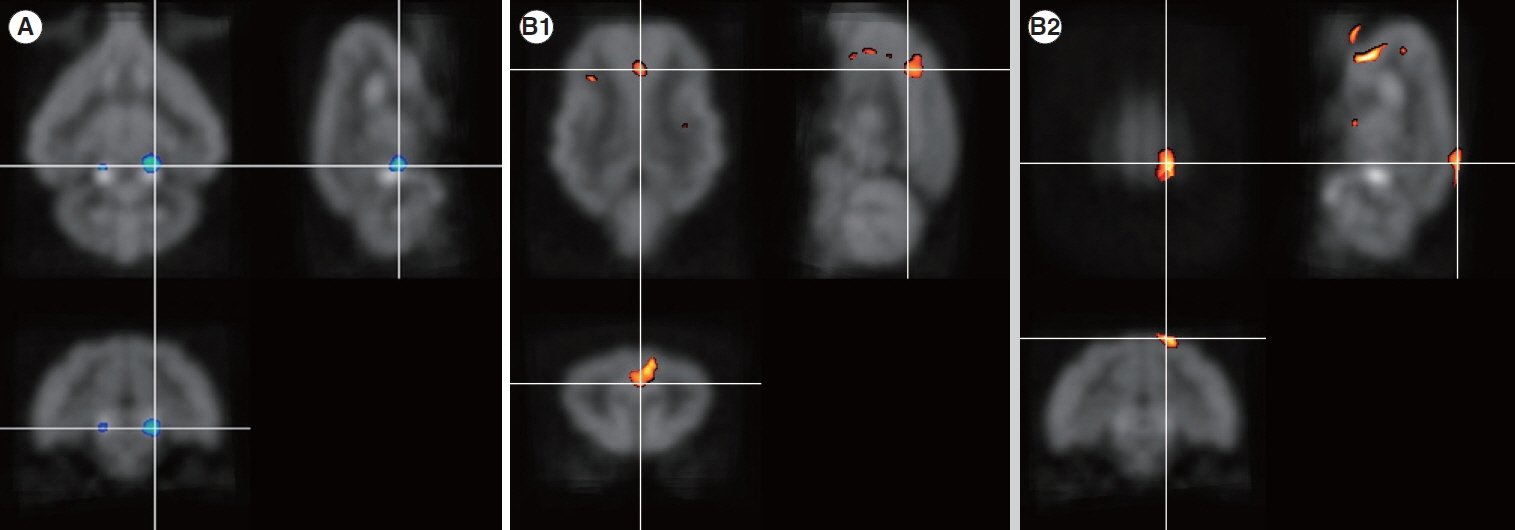
. Four months after hearing loss, the auditory cortical area’s activity decreased, and activity in the associated visual area increased. After 7 months of cochlear stimulation, the superior marginal gyrus and cingulate gyrus, which are components of the default mode network, showed hypermetabolism. The inferior colliculi showed hypometabolism.
Conclusion
. Resting-state cortical activity in the default mode network components was elevated after cochlear stimulation. This suggests that the animals’ awareness level was elevated after hearing restoration by the cochlear implantation.
Keyword
Figure
Reference
-
1. Sandmann P, Plotz K, Hauthal N, de Vos M, Schonfeld R, Debener S. Rapid bilateral improvement in auditory cortex activity in postlingually deafened adults following cochlear implantation. Clin Neurophysiol. 2015; Mar. 126(3):594–607.2. Strelnikov K, Marx M, Lagleyre S, Fraysse B, Deguine O, Barone P. PET-imaging of brain plasticity after cochlear implantation. Hear Res. 2015; Apr. 322:180–7.3. Chen LC, Stropahl M, Schonwiesner M, Debener S. Enhanced visual adaptation in cochlear implant users revealed by concurrent EEGfNIRS. Neuroimage. 2017; Feb. 146:600–8.4. Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012; Feb. 35(2):111–22.5. Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, et al. Spoken language development in children following cochlear implantation. JAMA. 2010; Apr. 303(15):1498–506.6. Suh MW, Lee HJ, Kim JS, Chung CK, Oh SH. Speech experience shapes the speechreading network and subsequent deafness facilitates it. Brain. 2009; Oct. 132(Pt 10):2761–71.7. Saliba J, Bortfeld H, Levitin DJ, Oghalai JS. Functional near-infrared spectroscopy for neuroimaging in cochlear implant recipients. Hear Res. 2016; Aug. 338:64–75.8. Lee JS, Lee DS, Oh SH, Kim CS, Kim JW, Hwang CH, et al. PET evidence of neuroplasticity in adult auditory cortex of postlingual deafness. J Nucl Med. 2003; Sep. 44(9):1435–9.9. Kim E, Kang H, Han KH, Lee HJ, Suh MW, Song JJ, et al. Reorganized brain white matter in early- and late-onset deafness with diffusion tensor imaging. Ear Hear. 2021; Jan/Feb. 42(1):223–34.10. Park MH, Lee HJ, Kim JS, Lee JS, Lee DS, Oh SH. Cross-modal and compensatory plasticity in adult deafened cats: a longitudinal PET study. Brain Res. 2010; Oct. 1354:85–90.11. Kral A. Auditory critical periods: a review from system’s perspective. Neuroscience. 2013; Sep. 247:117–33.12. Zhang LI, Bao S, Merzenich MM. Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci U S A. 2002; Feb. 99(4):2309–14.13. Kim H, Kang WS, Park HJ, Lee JY, Park JW, Kim Y, et al. Cochlear implantation in postlingually deaf adults is time-sensitive towards positive outcome: prediction using advanced machine learning techniques. Sci Rep. 2018; Dec. 8(1):18004.14. Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, et al. Cross-modal plasticity and cochlear implants. Nature. 2001; Jan. 409(6817):149–50.15. Han JH, Lee HJ, Kang H, Oh SH, Lee DS. Brain plasticity can predict the cochlear implant outcome in adult-onset deafness. Front Hum Neurosci. 2019; Feb. 13:38.16. Abrahamse R, Beynon A, Piai V. Long-term auditory processing outcomes in early implanted young adults with cochlear implants: the mismatch negativity vs. P300 response. Clin Neurophysiol. 2021; Jan. 132(1):258–68.17. Lee HJ, Giraud AL, Kang E, Oh SH, Kang H, Kim CS, et al. Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex. 2007; Apr. 17(4):909–17.18. Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 2003; Nov. 20(3):1894–8.19. Luan Y, Wang C, Jiao Y, Tang T, Zhang J, Teng GJ. Dysconnectivity of multiple resting-state networks associated with higher-order functions in sensorineural hearing loss. Front Neurosci. 2019; Feb. 13:55.20. Schulte A, Thiel CM, Gieseler A, Tahden M, Colonius H, Rosemann S. Reduced resting state functional connectivity with increasing agerelated hearing loss and McGurk susceptibility. Sci Rep. 2020; Oct. 10(1):16987.21. Jang JH, Lee HS, Oh SH, Park MH. Efficacy of the cat deafening method: co-administration of ethacrynic acid and kanamycin. Acta Otolaryngol. 2016; 136(3):289–92.22. Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993; Nov. 70(2):205–15.23. Kretzmer EA, Meltzer NE, Haenggeli CA, Ryugo DK. An animal model for cochlear implants. Arch Otolaryngol Head Neck Surg. 2004; May. 130(5):499–508.24. Kim JS, Yu AR, Kim KM, Oh SJ, Ryu JS, Kim HJ, et al. Validation of a postinjection transmission method for actual rat brain PET. Med Phys. 2012; Sep. 39(9):5614–20.25. Gray-Edwards HL, Salibi N, Josephson EM, Hudson JA, Cox NR, Randle AN, et al. High resolution MRI anatomy of the cat brain at 3 Tesla. J Neurosci Methods. 2014; Apr. 227:10–7.26. Stolzberg D, Wong C, Butler BE, Lomber SG. Catlas: an magnetic resonance imaging-based three-dimensional cortical atlas and tissue probability maps for the domestic cat (Felis catus). J Comp Neurol. 2017; Oct. 525(15):3190–206.27. Kim JS, Lee JS, Park MH, Kang H, Lee JJ, Lee HJ, et al. Assessment of cerebral glucose metabolism in cat deafness model: strategies for improving the voxel-based statistical analysis for animal PET studies. Mol Imaging Biol. 2008; 10(3):154–61.28. Green KM, Julyan PJ, Hastings DL, Ramsden RT. Auditory cortical activation and speech perception in cochlear implant users: effects of implant experience and duration of deafness. Hear Res. 2005; Jul. 205(1-2):184–92.29. Green KM, Julyan PJ, Hastings DL, Ramsden RT. Auditory cortical activation and speech perception in cochlear implant users. J Laryngol Otol. 2008; Mar. 122(3):238–45.30. Naito Y, Tateya I, Fujiki N, Hirano S, Ishizu K, Nagahama Y, et al. Increased cortical activation during hearing of speech in cochlear implant users. Hear Res. 2000; May. 143(1-2):139–46.31. Yoshida H, Takahashi H, Kanda Y, Chiba K. PET-CT observations of cortical activity in pre-lingually deaf adolescent and adult patients with cochlear implantation. Acta Otolaryngol. 2017; May. 137(5):464–70.32. Strelnikov K, Rouger J, Demonet JF, Lagleyre S, Fraysse B, Deguine O, et al. Does brain activity at rest reflect adaptive strategies? Evidence from speech processing after cochlear implantation. Cereb Cortex. 2010; May. 20(5):1217–22.33. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001; Jan. 98(2):676–82.34. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015; Jul. 38:433–47.35. Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007; May. 447(7140):83–6.36. Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012; Mar. 109(10):3979–84.37. Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, Mihalas S, et al. Large-scale topology and the default mode network in the mouse connectome. Proc Natl Acad Sci U S A. 2014; Dec. 111(52):18745–50.38. Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009; Jan. 29(4):1191–201.39. Belkhiria C, Vergara RC, San Martin S, Leiva A, Marcenaro B, Martinez M, et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Front Aging Neurosci. 2019; Apr. 11:97.40. Luan Y, Wang C, Jiao Y, Tang T, Zhang J, Lu C, et al. Abnormal functional connectivity and degree centrality in anterior cingulate cortex in patients with long-term sensorineural hearing loss. Brain Imaging Behav. 2020; Jun. 14(3):682–95.41. Eckert MA, Teubner-Rhodes S, Vaden KI. Is listening in noise worth it? The neurobiology of speech recognition in challenging listening conditions. Ear Hear. 37 Suppl 1(Suppl 1):101S–110S.