Restor Dent Endod.
2021 Nov;46(4):e48. 10.5395/rde.2021.46.e48.
Bone repair in defects filled with AH Plus sealer and different concentrations of MTA: a study in rat tibiae
- Affiliations
-
- 1Department of Oral & Maxillofacial Surgery and Periodontology, São Paulo University, School of Dentistry, Ribeirão Preto, SP, Brazil
- 2Department of Endodontics, Dental School, Uninovafapi University Center, Teresina, PI, Brazil
- 3Department of Endodontics, São Leopoldo Mandic Dental School, Campinas, SP, Brazil
- 4Department of Morphology, Health Science Center, State University of Piauí, Teresina, PI, Brazil
- KMID: 2548092
- DOI: http://doi.org/10.5395/rde.2021.46.e48
Abstract
Objectives
This study aimed to evaluate the effects on bone repair of different concentrations of mineral trioxide aggregate (MTA) added to AH Plus.
Materials and Methods
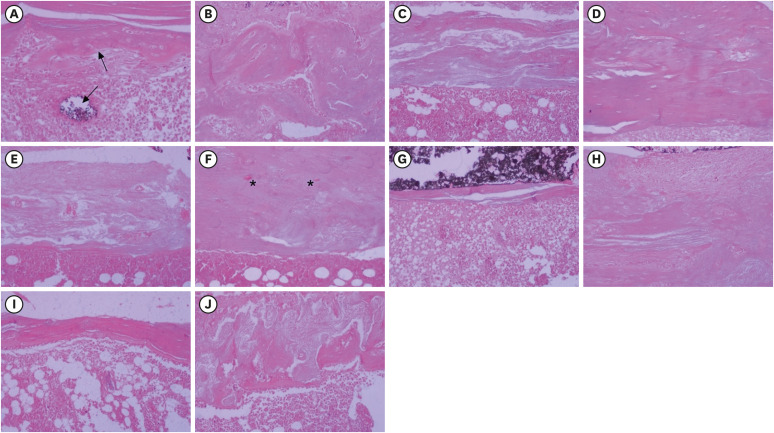
Bone tissue reactions were evaluated in 30 rats (Rattus norvegicus) after 7 and 30 days. In the AH + MTA10, AH + MTA20, and AH + MTA30 groups, defects in the tibiae were filled with AH Plus with MTA in proportions of 10%, 20% and 30%, respectively; in the MTA-FILL group, MTA Fillapex was used; and in the control group, no sealer was used. The samples were histologically analyzed to assess bone union and maturation. The Kruskal-Wallis and Mann-Whitney tests were performed for multiple pairwise comparisons (p ≤ 0.05).
Results
At the 7-day time point, AH + MTA10 was superior to MTA-FILL with respect to bone union, and AH + MTA20 was superior to MTA-FILL with respect to bone maturity (p < 0.05). At the 30-day time point, both the AH + MTA10 and AH + MTA20 experimental sealers were superior not only to MTA-FILL, but also to AH + MTA30 with respect to both parameters (p < 0.05). The results of the AH + MTA10 and AH + MTA20 groups were superior to those of the control group for both parameters and experimental time points (p < 0.05).
Conclusions
The results suggest the potential benefit of using a combination of these materials in situations requiring bone repair.
Keyword
Figure
Reference
-
1. Lim M, Jung C, Shin DH, Cho YB, Song M. Calcium silicate-based root canal sealers: a literature review. Restor Dent Endod. 2020; 45:e35. PMID: 32839716.
Article2. Fonseca DA, Paula AB, Marto CM, Coelho A, Paulo S, Martinho JP, Carrilho E, Ferreira MM. Biocompatibility of root canal sealers: a systematic review of in vitro and in vivo studies. Materials (Basel). 2019; 12:4113–4117.
Article3. Al-Haddad A, Che Ab Aziz ZA. Bioceramic-based root canal sealers: a review. Int J Biomater. 2016; 2016:9753210. PMID: 27242904.
Article4. Kaur A, Shah N, Logani A, Mishra N. Biotoxicity of commonly used root canal sealers: a meta-analysis. J Conserv Dent. 2015; 18:83–88. PMID: 25829682.
Article5. Jafari F, Jafari S. Composition and physicochemical properties of calcium silicate based sealers: a review article. J Clin Exp Dent. 2017; 9:e1249–e1255. PMID: 29167717.
Article6. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995; 21:349–353. PMID: 7499973.
Article7. Torabinejad M, Parirokh M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J. 2018; 51:284–317. PMID: 28846134.
Article8. Assmann E, Böttcher DE, Hoppe CB, Grecca FS, Kopper PM. Evaluation of bone tissue response to a sealer containing mineral trioxide aggregate. J Endod. 2015; 41:62–66. PMID: 25447498.
Article9. Jung S, Sielker S, Hanisch MR, Libricht V, Schäfer E, Dammaschke T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS One. 2018; 13:e0194467. PMID: 29579090.
Article10. Kebudi Benezra M, Schembri Wismayer P, Camilleri J. Interfacial characteristics and cytocompatibility of hydraulic sealer cements. J Endod. 2018; 44:1007–1017. PMID: 29398087.
Article11. Cintra LTA, Benetti F, de Azevedo Queiroz ÍO, Ferreira LL, Massunari L, Bueno CRE, de Oliveira SHP, Gomes-Filho JE. Evaluation of the cytotoxicity and biocompatibility of new resin epoxy-based endodontic sealer containing calcium hydroxide. J Endod. 2017; 43:2088–2092. PMID: 29032822.
Article12. de Oliveira RL, Oliveira Filho RS, Gomes HC, de Franco MF, Enokihara MM, Duarte MA. Influence of calcium hydroxide addition to AH Plus sealer on its biocompatibility. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:e50–e54. PMID: 20123371.
Article13. Falcão LF, Silva PR, Falcão DF, Pinto LSS, Falcão CAM. Evaluation of the biocompatibility of AH Plus sealer associated to Portland cement or MTA. Subcutaneous study in rats. Rev Interd. 2018; 11:1–11.14. Garcia LF, Huck C, Scardueli CR, de Souza Costa CA. Repair of bone defects filled with new calcium aluminate cement (EndoBinder). J Endod. 2015; 41:864–870. PMID: 25720982.
Article15. Falcão CA, Lima EM, Júnior JDM, Freitas SA, Veras ESL, Moura LK, Falcão LF. Plus adhesiveness assessment associated with mineral trioxide aggregate in different proportions (push-out test). J Contemp Dent Pract. 2018; 19:1444–1447. PMID: 30713171.16. Hedner E, Linde A. Efficacy of bone morphogenetic protein (BMP) with osteopromotive membranes--an experimental study in rat mandibular defects. Eur J Oral Sci. 1995; 103:236–241. PMID: 7552955.
Article17. Delfino MM, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Sasso-Cerri E, Cerri PS. Immunoinflammatory response and bioactive potential of GuttaFlow bioseal and MTA Fillapex in the rat subcutaneous tissue. Sci Rep. 2020; 10:7173. PMID: 32346066.
Article18. Sousa Filho JL, Moreira KMS, Amaral GCLS, Fortunato CFP, Dias IVA, Falcão CAM. Radiopacity of AH Plus endodontic sealer plus MTA and Portland cement. Dental Press Endod. 2018; 8:18–21.19. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005; 31:97–100. PMID: 15671817.
Article20. Costa F, Sousa Gomes P, Fernandes MH. Osteogenic and angiogenic response to calcium silicate–based endodontic sealers. J Endod. 2016; 42:113–119. PMID: 26577874.
Article21. Mestieri LB, Zaccara IM, Pinheiro LS, Barletta FB, Kopper PMP, Grecca FS. Cytocompatibility and cell proliferation evaluation of calcium phosphate-based root canal sealers. Restor Dent Endod. 2019; 45:e2. PMID: 32110532.
Article22. Salles LP, Gomes-Cornélio AL, Guimarães FC, Herrera BS, Bao SN, Rossa-Junior C, Guerreiro-Tanomaru JM, Tanomaru-Filho M. Mineral trioxide aggregate-based endodontic sealer stimulates hydroxyapatite nucleation in human osteoblast-like cell culture. J Endod. 2012; 38:971–976. PMID: 22703663.
Article23. Saraiva JA, da Fonseca TS, da Silva GF, Sasso-Cerri E, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Cerri PS. Reduced interleukin-6 immunoexpression and birefringent collagen formation indicate that MTA Plus and MTA Fillapex are biocompatible. Biomed Mater. 2018; 13:035002. PMID: 29242419.
Article24. Scelza MZ, Campos CA, Scelza P, Adeodato CS, Barbosa IB, de Noronha F, Montalli V, Napimoga M, de Araújo VC, Alves GG. Evaluation of inflammatory response to endodontic sealers in a bone defect animal model. J Contemp Dent Pract. 2016; 17:536–541. PMID: 27595718.
Article25. Almeida LH, Gomes APN, Gastmann AH, Pola NM, Moraes RR, Morgental RD, Cava SS, Felix AOC, Pappen FG. Bone tissue response to an MTA-based endodontic sealer, and the effect of the addition of calcium aluminate and silver particles. Int Endod J. 2019; 52:1446–1456. PMID: 31034099.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytocompatibility and cell proliferation evaluation of calcium phosphate-based root canal sealers
- A micro-computed tomographic evaluation of root canal filling with a single gutta-percha cone and calcium silicate sealer
- Micro-computed tomographic evaluation of single-cone obturation with three sealers
- A micro-computed tomographic study of remaining filling materials of two bioceramic sealers and epoxy resin sealer after retreatment
- Biological assessment of a new readyto-use hydraulic sealer