J Korean Med Sci.
2023 Nov;38(44):e350. 10.3346/jkms.2023.38.e350.
Antenatal Magnesium Sulfate Is Not Associated With Improved Long-Term Neurodevelopment and Growth in Very Low Birth Weight Infants
- Affiliations
-
- 1Department of Pediatrics, Inha University Hospital, Inha University College of Medicine, Incheon, Korea
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Sciences & Technology (SAIHST), Sungkyunkwan University, Seoul, Korea
- 4Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences & Technology (SAIHST), Samsung Medical Center, Seoul, Korea
- KMID: 2547970
- DOI: http://doi.org/10.3346/jkms.2023.38.e350
Abstract
- Background
Though antenatal magnesium sulfate (MgSO4 ) is widely used for fetal neuroprotection, suspicions about the long-term neuroprotection of antenatal MgSO4 have been raised.
Methods
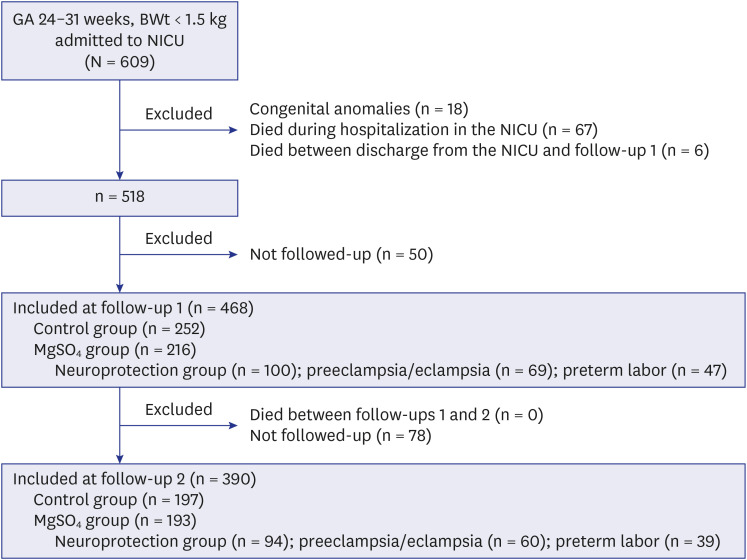
We investigated short- and long-term outcomes of antenatal MgSO4 use for 468 infants weighing < 1,500 g with a gestational age of 24–31 weeks.
Results
Short-term morbidities and the risk of developmental delay, hearing loss, and cerebral palsy at a corrected age of 18–24 months and 3 years of age did not decrease in the MgSO4 group (infants who were exposed to MgSO4 for any purpose) or neuroprotection group (infants who were exposed to MgSO4 for fetal neuroprotection) compared with the control group (infants who were not exposed to MgSO4 ). The z-scores of weight, height, and head circumference did not increase in the MgSO4 group or neuroprotection group compared with the control group.
Conclusion
Antenatal MgSO4 including MgSO4 for neuroprotection did not have beneficial effects on long-term neurodevelopmental and growth outcomes.
Keyword
Figure
Reference
-
1. McNamara HC, Crowther CA, Brown J. Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour. Cochrane Database Syst Rev. 2015; 2015(12):CD011200. PMID: 26662716.
Article2. Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009; (1):CD004661. PMID: 19160238.
Article3. Costantine MM, Weiner SJ. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal–Fetal Medicine Units Network (MFMU). Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet Gynecol. 2009; 114(2 Pt 1):354–364. PMID: 19622997.
Article4. Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am J Obstet Gynecol. 2009; 200(6):595–609. PMID: 19482113.
Article5. Committee opinion No. 455: magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol. 2010; 115(3):669–671. PMID: 20177305.6. American College of Obstetricians and Gynecologists. Society for Maternal-Fetal Medicine. Obstetric care consensus No. 6: periviable birth. Obstet Gynecol. 2017; 130(4):e187–e199. PMID: 28937572.7. Magee LA, De Silva DA, Sawchuck D, Synnes A, von Dadelszen P. No. 376-magnesium sulphate for fetal neuroprotection. J Obstet Gynaecol Can. 2019; 41(4):505–522. PMID: 30879485.
Article8. Crowther CA, Hiller JE, Doyle LW, Haslam RR. Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003; 290(20):2669–2676. PMID: 14645308.
Article9. Doyle LW, Anderson PJ, Haslam R, Lee KJ, Crowther C. Australasian Collaborative Trial of Magnesium Sulphate (ACTOMgSO4) Study Group. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA. 2014; 312(11):1105–1113. PMID: 25226476.
Article10. Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot MF, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG. 2007; 114(3):310–318. PMID: 17169012.
Article11. Chollat C, Enser M, Houivet E, Provost D, Bénichou J, Marpeau L, et al. School-age outcomes following a randomized controlled trial of magnesium sulfate for neuroprotection of preterm infants. J Pediatr. 2014; 165(2):398–400.e3. PMID: 24837863.
Article12. Garg BD. Antenatal magnesium sulfate is beneficial or harmful in very preterm and extremely preterm neonates: a new insight. J Matern Fetal Neonatal Med. 2019; 32(12):2084–2090. PMID: 29301419.
Article13. Hong JY, Hong JY, Choi YS, Kim YM, Sung JH, Choi SJ, et al. Antenatal magnesium sulfate treatment and risk of necrotizing enterocolitis in preterm infants born at less than 32 weeks of gestation. Sci Rep. 2020; 10(1):12826. PMID: 32733081.
Article14. Sung SI, Ahn SY, Choi SJ, Oh SY, Roh CR, Yang M, et al. Increased risk of meconium-related ileus in extremely premature infants exposed to antenatal magnesium sulfate. Neonatology. 2022; 119(1):68–76. PMID: 35016173.
Article15. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33(1):179–201. PMID: 3081865.
Article16. Jeon GW, Oh M, Lee J, Jun YH, Chang YS. Comparison of definitions of bronchopulmonary dysplasia to reflect the long-term outcomes of extremely preterm infants. Sci Rep. 2022; 12(1):18095. PMID: 36302832.
Article17. Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part II. Ann Neurol. 1989; 25(2):109–116. PMID: 2645823.
Article18. Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International Classification of Retinopathy of Prematurity, third edition. Ophthalmology. 2021; 128(10):e51–e68. PMID: 34247850.
Article19. Yoon SA, Lee MH, Chang YS. Impact of time to full enteral feeding on long-term neurodevelopment without mediating by postnatal growth failure in very-low-birth-weight-infants. Sci Rep. 2023; 13(1):2990. PMID: 36804430.
Article20. Chung HJ, Yang D, Kim GH, Kim SK, Kim SW, Kim YK, et al. Development of the Korean Developmental Screening Test for Infants and Children (K-DST). Clin Exp Pediatr. 2020; 63(11):438–446. PMID: 32683817.
Article21. Kim CY, Jung E, Lee BS, Kim KS, Kim EA. Validity of the Korean Developmental Screening Test for very-low-birth-weight infants. Korean J Pediatr. 2019; 62(5):187–192. PMID: 30999730.
Article22. Yim CH, Kim GH, Eun BL. Usefulness of the Korean Developmental Screening Test for infants and children for the evaluation of developmental delay in Korean infants and children: a single-center study. Korean J Pediatr. 2017; 60(10):312–319. PMID: 29158765.
Article23. Begnoche DM, Chiarello LA, Palisano RJ, Gracely EJ, McCoy SW, Orlin MN. Predictors of independent walking in young children with cerebral palsy. Phys Ther. 2016; 96(2):183–192. PMID: 26089044.
Article24. WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006; 450:76–85. PMID: 16817681.25. Marret S, Marpeau L, Follet-Bouhamed C, Cambonie G, Astruc D, Delaporte B, et al. Effect of magnesium sulphate on mortality and neurologic morbidity of the very-preterm newborn (of less than 33 weeks) with two-year neurological outcome: results of the prospective PREMAG trial. Gynecol Obstet Fertil. 2008; 36(3):278–288. PMID: 18337147.
Article26. Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008; 359(9):895–905. PMID: 18753646.
Article27. Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002; 186(6):1111–1118. PMID: 12066082.
Article28. Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG. 2007; 114(3):289–299. PMID: 17166221.29. Zeng X, Xue Y, Tian Q, Sun R, An R. Effects and safety of magnesium sulfate on neuroprotection: a meta-analysis based on PRISMA guidelines. Medicine (Baltimore). 2016; 95(1):e2451. PMID: 26735551.30. Poppe T, Thompson B, Boardman JP, Bastin ME, Alsweiler J, Deib G, et al. Effect of antenatal magnesium sulphate on MRI biomarkers of white matter development at term equivalent age: the MagNUM study. EBioMedicine. 2022; 78:103923. PMID: 35331677.
Article31. Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: an individual participant data meta-analysis. PLoS Med. 2017; 14(10):e1002398. PMID: 28976987.
Article32. Wolf HT, Huusom LD, Henriksen TB, Hegaard HK, Brok J, Pinborg A. Magnesium sulphate for fetal neuroprotection at imminent risk for preterm delivery: a systematic review with meta-analysis and trial sequential analysis. BJOG. 2020; 127(10):1180–1188. PMID: 32237069.
Article33. Chollat C, Bertrand E, Petit-Ledo A, de Vansay C, Voisin C, Dabaj I, et al. Cerebral palsy in very preterm infants: a nine-year prospective study in a French population-based tertiary center. J Pediatr. 2021; 237:183–189.e6. PMID: 34144033.
Article34. Ahn JH, Lee KM, Kim MJ, Park HK, Kim YJ, Ahn SJ, et al. Neurodevelopmental outcomes in very low birthweight infants with retinopathy of prematurity in a nationwide cohort study. Sci Rep. 2022; 12(1):5053. PMID: 35322163.
Article35. Han G, Lim DH, Kang D, Cho J, Guallar E, Chang YS, et al. Association between retinopathy of prematurity in very-low-birth-weight infants and neurodevelopmental impairment. Am J Ophthalmol. 2022; 244:205–215. PMID: 35998681.
Article36. Yi YG, Sung IY, Yuk JS. Comparison of second and third editions of the bayley scales in children with suspected developmental delay. Ann Rehabil Med. 2018; 42(2):313–320. PMID: 29765885.
Article37. Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014; 75(5):670–674. PMID: 24492622.
Article38. Moser MF, Müller IJ, Schalamon J, Resch B. Neurodevelopmental outcome of very preterm infants with gastrointestinal tract perforations does not differ compared to controls. Wien Klin Wochenschr. 2021; 133(13-14):680–686. PMID: 34110498.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Antenatal Exposure to Magnesium Sulfate on Neuroprotection in Preterm Infants
- Influence of Antenatal Magnesium Sulfate Exposure on Perinatal Outcomes in VLBW Infants with Maternal Preeclampsia
- Obstetrical Management of Periviable Birth
- Antenatal Magnesium Sulfate for Neuroprotective Effects In Preterm Infants
- Prognosis of Extremely Low Birth Weight infants (1,000 g) Associated with Antenatal Magnesium Sulfate (MgSO4) Exposure