J Pathol Transl Med.
2023 Nov;57(6):337-340. 10.4132/jptm.2023.09.22.
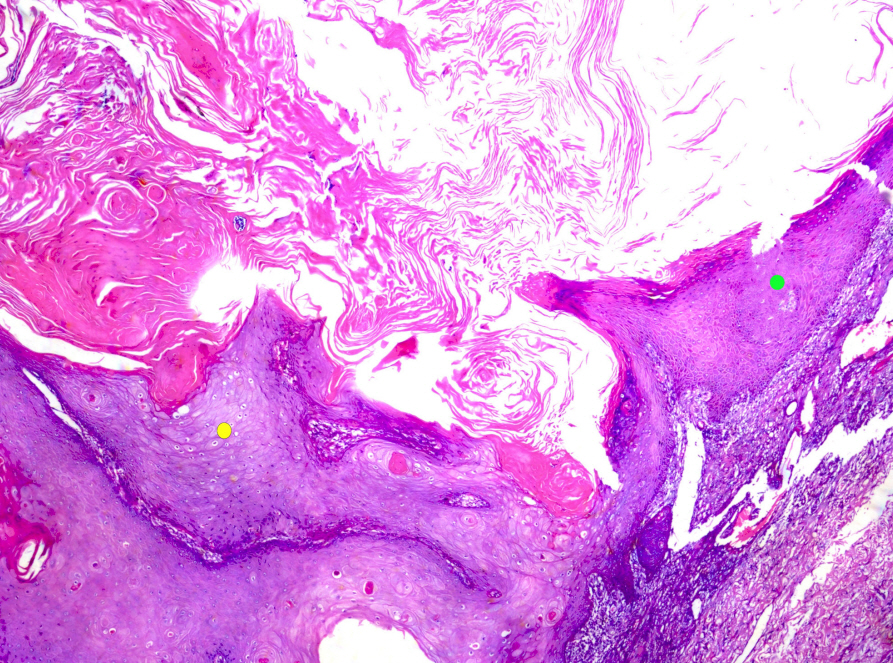
What’s new in dermatopathology 2023: WHO 5th edition updates
- Affiliations
-
- 1Department of Pathology, The University of the West Indies, Mona Campus, Jamaica W.I.
- 2Division of Dermatology, Department of Medicine, The University of the West Indies, Mona Campus, Jamaica W.I.
- KMID: 2547932
- DOI: http://doi.org/10.4132/jptm.2023.09.22
Abstract
- The 5th edition WHO Classification of Skin Tumors (2022) has introduced changes to nomenclature and diagnostics. Important differences are discussed below. Changes in each category of skin tumor have been detailed, with particular emphasis on meaningful advances in our understanding of the molecular pathogenesis of the skin’s diverse tumor landscape.
Figure
Reference
-
References
1. Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014; 9:239–71.
Article2. Raghavan SS, Saleem A, Wang JY, Rieger KE, Brown RA, Novoa RA. Diagnostic utility of LEF1 immunohistochemistry in differentiating deep penetrating nevi from histologic mimics. Am J Surg Pathol. 2020; 44:1413–8.
Article3. de la Fouchardiere A, Pissaloux D, Tirode F, Karanian M, Fletcher CDM, Hanna J. Clear cell tumor with melanocytic differentiation and ACTIN-MITF translocation: report of 7 cases of a novel entity. Am J Surg Pathol. 2021; 45:962–8.4. de la Fouchardiere A, Pissaloux D, Tirode F, Hanna J. Clear cell tumor with melanocytic differentiation and MITF-CREM translocation: a novel entity similar to clear cell sarcoma. Virchows Arch. 2021; 479:841–6.
Article5. Sekine S, Kiyono T, Ryo E, et al. Recurrent YAP1- MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Invest. 2019; 129:3827–32.6. Nishimura Y, Ryo E, Yamazaki N, Yatabe Y, Mori T. Cutaneous primary NUT carcinoma With BRD3- NUTM1 Fusion. Am J Surg Pathol. 2021; 45:1582–4.7. Héritier S, Emile JF, Barkaoui MA, et al. BRAF mu- tation correlates with high-risk langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol. 2016; 34:3023–30.8. Bontoux C, Baroudjian B, Le Maignan C, et al. CRTC1-TRIM11 fusion in a case of metastatic clear cell sarcoma: are CRTC1-TRIM11 fusion-bearing tumors melanocytomas or clear cell sarcomas? Am J Surg Pathol. 2019; 43:861–3.9. Yang L, Yin Z, Wei J, et al. Cutaneous melanocytic tumour with CRTC1::TRIM11 fusion in a case with recurrent local lymph node and distant pulmonary metastases at early stage: aggressive rather than indolent? Histopathology. 2023; 82:368–71.
Article10. Puls F, Carter JM, Pillay N, et al. Overlapping morphological, immunohistochemical and genetic features of superficial CD34-positive fibroblastic tumor and PRDM10-rearranged soft tissue tumor. Mod Pathol. 2022; 35:767–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What’s new in kidney tumor pathology 2022: WHO 5th edition updates
- What’s new in hematopathology 2023: updates on mature T-cell neoplasms in the 5th edition of the WHO classification
- What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis
- What’s new in neuropathology 2024: CNS WHO 5th edition updates
- What’s new in breast pathology 2022: WHO 5th edition and biomarker updates