J Pathol Transl Med.
2023 Nov;57(6):323-331. 10.4132/jptm.2023.10.22.
BRCA-mutated gastric adenocarcinomas are associated with chromosomal instability and responsiveness to platinum-based chemotherapy
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Medical Science, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Asan Center for Cancer Genome Discovery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2547930
- DOI: http://doi.org/10.4132/jptm.2023.10.22
Abstract
- Background
Homologous recombination defect is an important biomarker of chemotherapy in certain tumor types, and the presence of pathogenic or likely pathogenic mutations involving BRCA1 or BRCA2 (p-BRCA) mutations is the most well-established marker for the homologous recombination defect. Gastric cancer, one of the most prevalent tumor types in Asia, also harbors p-BRCA mutations.
Methods
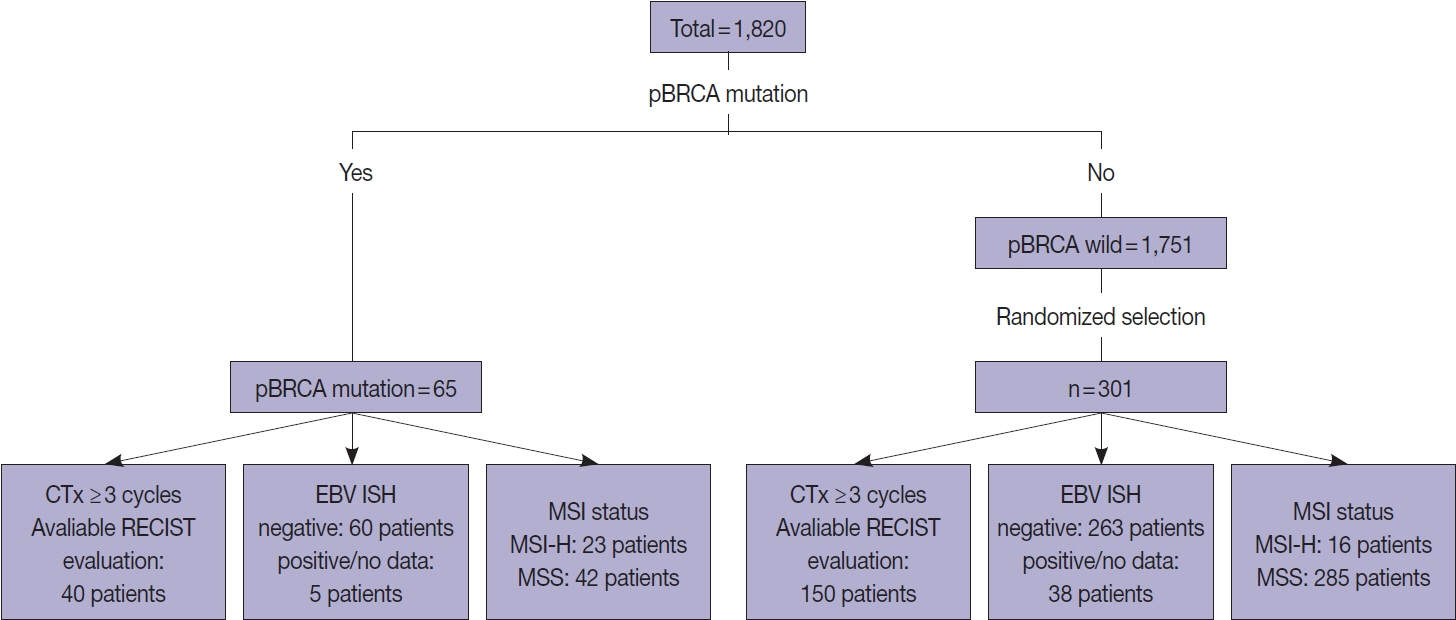
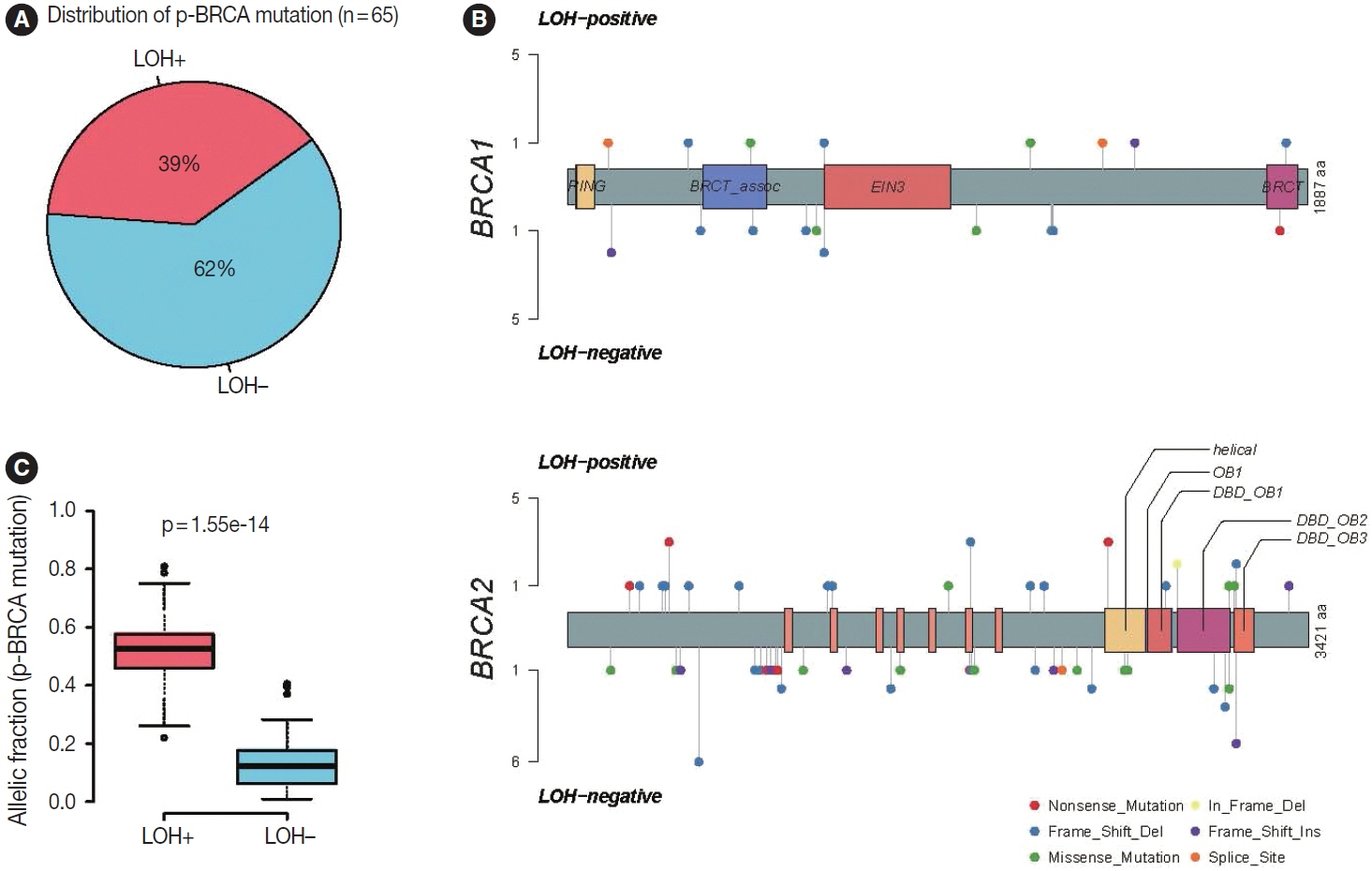
To investigate the clinical significance of p-BRCA mutations, we analyzed 366 gastric cancer cases through next-generation sequencing. We determined the zygosity of p-BRCA mutations based on the calculated tumor purity through variant allelic fraction patterns and investigated whether the presence of p-BRCA mutations is associated with platinum-based chemotherapy and a certain molecular subtype.
Results
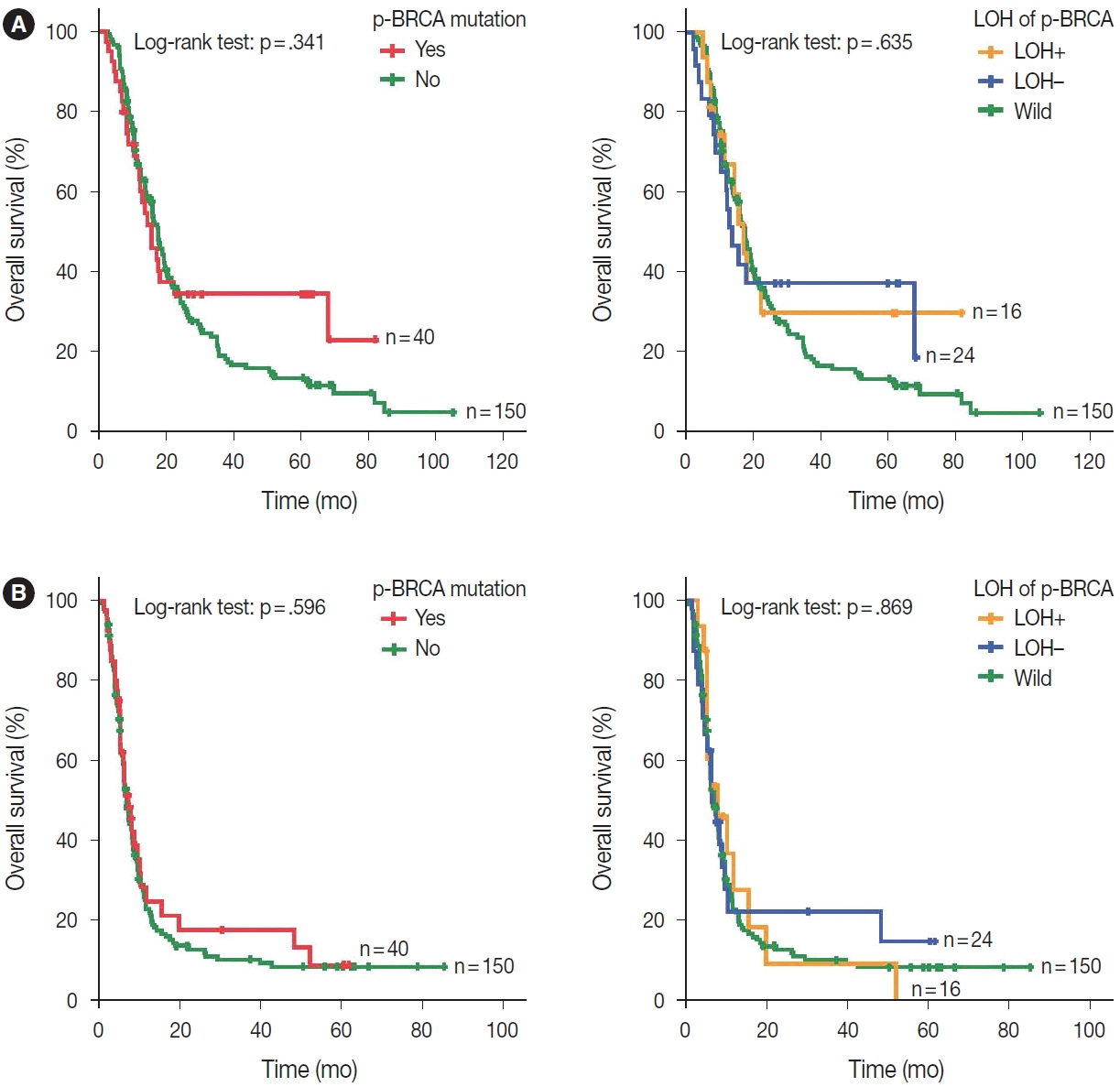
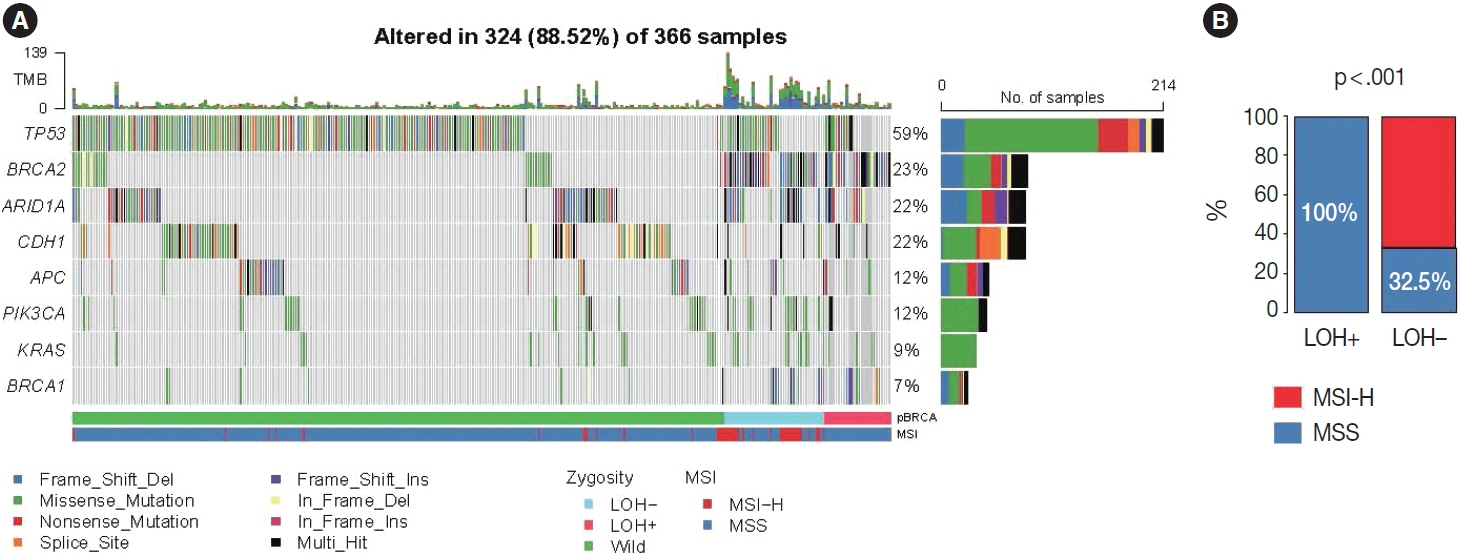
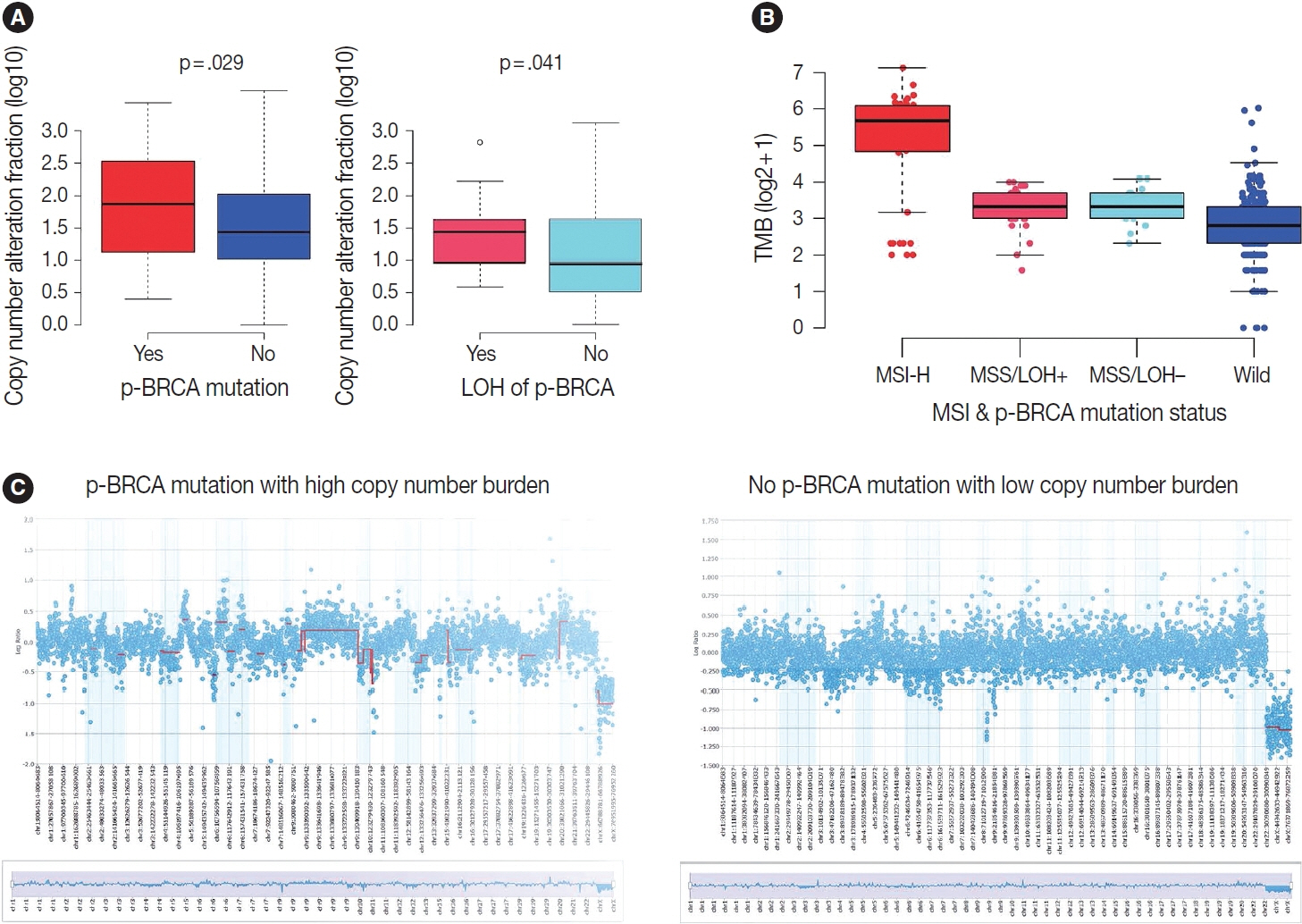
Biallelic p-BRCA mutation was associated with better response to platinum-based chemotherapy than heterozygous p-BRCA mutation or wild type BRCA genes. The biallelic p-BRCA mutations was observed only in the chromosomal instability subtype, while all p-BRCA mutations were heterozygous in microsatellite instability subtype.
Conclusions
In conclusion, patients with gastric cancer harboring biallelic p-BRCA mutations were associated with a good initial response to platinum-based chemotherapy and those tumors were exclusively chromosomal instability subtype. Further investigation for potential association with homologous recombination defect is warranted.
Keyword
Figure
Cited by 1 articles
-
Presence of
RB1 or Absence ofLRP1B Mutation Predicts Poor Overall Survival in Patients with Gastric Neuroendocrine Carcinoma and Mixed Adenoneuroendocrine Carcinoma
In Hye Song, Bokyung Ahn, Young Soo Park, Deok Hoon Kim, Seung-Mo Hong
Cancer Res Treat. 2025;57(2):492-506. doi: 10.4143/crt.2024.667.
Reference
-
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020; 396:635–48.
Article2. Park SH, Kang MJ, Yun EH, Jung KW. Epidemiology of gastric cancer in Korea: trends in incidence and survival based on Korea Central Cancer Registry data (1999-2019). J Gastric Cancer. 2022; 22:160–8.
Article3. Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep. 2019; 21:67.
Article4. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20.
Article5. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019; 393:1948–57.6. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022; 20:167–92.7. Yan D, Dai H. FOLFOX regimen in the patients with locally advanced or metastatic gastric cancer. Zhonghua Zhong Liu Za Zhi. 2009; 31:217–9.8. Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol. 2020; 21:70.
Article9. Zhang J, Willers H, Feng Z, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004; 24:708–18.
Article10. Mylavarapu S, Das A, Roy M. Role of BRCA mutations in the modulation of response to platinum therapy. Front Oncol. 2018; 8:16.
Article11. Kim D, Nam HJ. PARP inhibitors: clinical limitations and recent attempts to overcome them. Int J Mol Sci. 2022; 23:8412.
Article12. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019; 571:576–9.
Article13. Noh JM, Choi DH, Baek H, et al. Associations between BRCA mutations in high-risk breast cancer patients and familial cancers other than breast or ovary. J Breast Cancer. 2012; 15:283–7.
Article14. Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021; 19:77–102.15. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–9.16. Kim JE, Chun SM, Hong YS, et al. Mutation burden and I index for detection of microsatellite instability in colorectal cancer by targeted next-generation sequencing. J Mol Diagn. 2019; 21:241–50.
Article17. Kim M, Lee C, Hong J, et al. Validation and clinical application of ONCOaccuPanel for targeted next-generation sequencing of solid tumors. Cancer Res Treat. 2023; 55:429–41.
Article18. Buchhalter I, Rempel E, Endris V, et al. Size matters: Dissecting key parameters for panel-based tumor mutational burden analysis. Int J Cancer. 2019; 144:848–58.
Article19. Talevich E, Shain AH, Botton T, et al. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol. 2016; 12:e1004873.
Article20. Siegmund SE, Manning DK, Davineni PK, Dong F. Deriving tumor purity from cancer next generation sequencing data: applications for quantitative ERBB2 (HER2) copy number analysis and germline inference of BRCA1 and BRCA2 mutations. Mod Pathol. 2022; 35:1458–67.
Article21. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article22. Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018; 173:321–37.23. Wodarz D, Newell AC, Komarova NL. Passenger mutations can accelerate tumour suppressor gene inactivation in cancer evolution. J R Soc Interface. 2018; 15:20170967.
Article24. Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015; 16:87–97.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chromosomal Instability in the Peripheral Blood Lymphocytes of an Ovarian Cancer Patient Undergoing Chemotherapy
- Efficacy and impact of PARPi monotherapy to subsequent platinum-based chemotherapy in BRCA1/2 mutant ovarian cancer patients with secondary platinum-sensitive relapse
- A single-arm phase II study of olaparib maintenance with pembrolizumab and bevacizumab in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer (OPEB-01)
- Chemotherapy for Metastatic Gastric Cancer
- Genomic Instability in Colorectal Cancer; from Bench to Bed