Clin Endosc.
2023 Nov;56(6):812-816. 10.5946/ce.2022.117.
Gastric cancer presenting with ramucirumab-related gastrocolic fistula successfully managed by colonic stenting: a case report
- Affiliations
-
- 1Department of Gastroenterology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan
- 2Institute of Research Center, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan
- 3Department of Gastroenterology and Hepatology, National Hospital Organization Fukuoka Higashi Medical Center, Fukuoka, Japan
- 4Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- KMID: 2547904
- DOI: http://doi.org/10.5946/ce.2022.117
Abstract
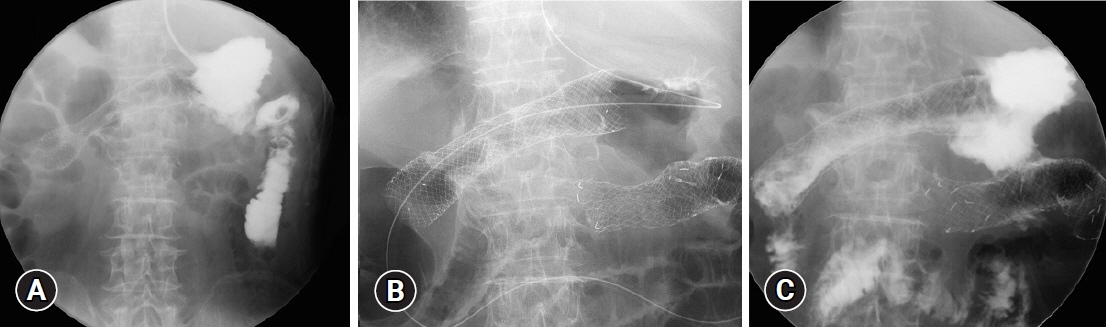
- We report a rare case of gastric cancer presenting with a gastrocolic fistula during ramucirumab and paclitaxel combination therapy that was successfully managed with colonic stenting. A 75-year-old man was admitted to our hospital with the chief complaint of melena. Esophagogastroduodenoscopy revealed a large ulcerated tumor in the lower stomach, judged by laparoscopy as unresectable (sT4bN1M0). After four cycles of first-line chemotherapy with S-1 plus oxaliplatin, the patient showed disease progression, and second-line therapy with ramucirumab and paclitaxel was started. At the end of the third cycle, the patient had gastric antral stenosis, which necessitated the placement of a gastroduodenal stent. When the patient complained of diarrhea 10 days later, esophagogastroduodenoscopy revealed a fistula between the greater curvature of the stomach and the transverse colon. The fistula was covered by double colonic stenting, with a covered metal stent placed within an uncovered metal stent, after which leakage from the stomach to the colon stopped.
Figure
Reference
-
1. Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010; 28:780–787.
Article2. Jung YD, Mansfield PF, Akagi M, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002; 38:1133–1140.
Article3. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–39.
Article4. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–1235.
Article5. Wang Z, Zhang J, Zhang L, et al. Risk of gastrointestinal perforation in cancer patients receiving ramucirumab: a meta-analysis of randomized controlled trials. J Chemother. 2016; 28:328–334.
Article6. Jung M, Ryu MH, Oh DY, et al. Efficacy and tolerability of ramucirumab monotherapy or in combination with paclitaxel in gastric cancer patients from the expanded access program cohort by the Korean Cancer Study Group (KCSG). Gastric Cancer. 2018; 21:819–830.
Article7. Marshall SF, Knud-Hansen J. Gastrojejunocolic and gastrocolic fistulas. Ann Surg. 1957; 145:770–782.
Article8. Amlicke JA, Ponka JL. Gastrocolic and gastrojejunocolic fistulas: a report of sixteen cases. Am J Surg. 1964; 107:744–750.9. Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007; 14:1860–1869.
Article10. Zheng Y, Gao W, Spratt DE, et al. Management of gastrointestinal perforation related to radiation. Int J Clin Oncol. 2020; 25:1010–1015.
Article11. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: prevention and treatment. World J Gastroenterol. 2013; 19:199–208.
Article12. ReMine SG, McIlrath DC. Bowel perforation in steroid-treated patients. Ann Surg. 1980; 192:581–586.
Article13. de Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol. 2001; 1:1.
Article14. Rogalski P, Daniluk J, Baniukiewicz A, et al. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015; 21:10542–10552.
Article15. Lim CH, Kim SW, Kim JS, et al. Successful palliation of a gastrocolic fistula secondary to gastric cancer by insertion of a covered colonic stent. Gastrointest Endosc. 2011; 73:1314–1317.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Gastrocolic Fistula Secondary to Colon Cancer
- A Case of Gastrocolic Fistula as a Complication of Colon Cancer
- Acute Ischemic Stroke in a Patient with Metastatic Gastric Cancer after Ramucirumab Chemotherapy
- A case of combined gastrojejunal and gastrocolic fistula secondary to gastric cancer
- An Unusual Cause of Gastrointestinal Hemorrhage: Gastrocolic Fistula Caused by Colon Cancer Invasion