Cancer Res Treat.
2023 Oct;55(4):1144-1151. 10.4143/crt.2023.403.
Expanded Access Program Pralsetinib in Advanced Non–Small Cell Lung Cancer with Rearranged during Transfection (RET) Gene Rearrangement
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2547789
- DOI: http://doi.org/10.4143/crt.2023.403
Abstract
- Purpose
Rearranged during transfection (RET) gene rearrangement is a well-known driver event in non–small cell lung cancer (NSCLC). Pralsetinib is a selective inhibitor of RET kinase and has shown efficacy in oncogenic RET-altered tumors. This study evaluated the efficacy and safety of expanded access program (EAP) use of pralsetinib in pretreated, advanced NSCLC patients with RET rearrangement.
Materials and Methods
Patients who received pralsetinib as part of the EAP at Samsung Medical Center were evaluated through a retrospective chart review. The primary endpoint was overall response rate (ORR) per the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 guidelines. Secondary endpoints were duration of response, progression-free survival (PFS), overall survival (OS), and safety profiles.
Results
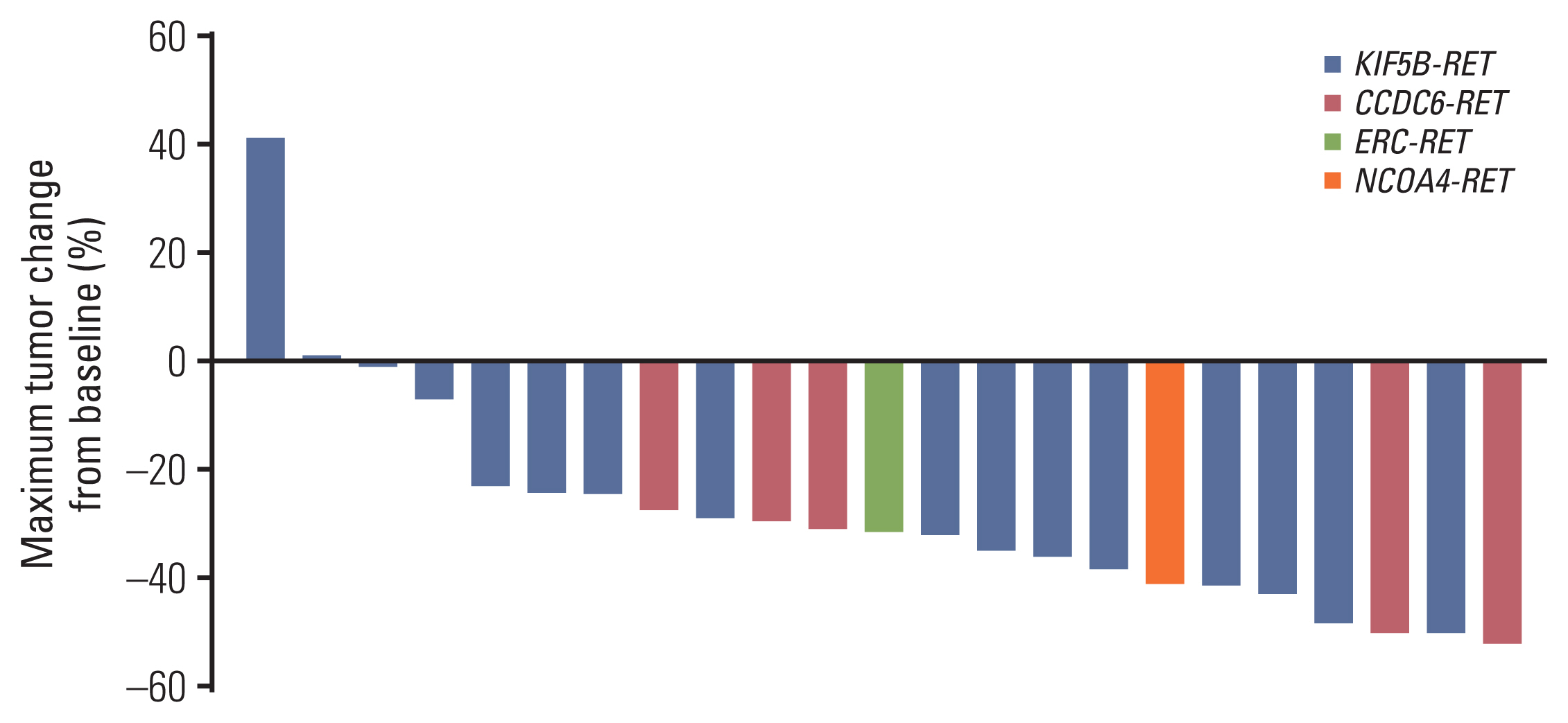
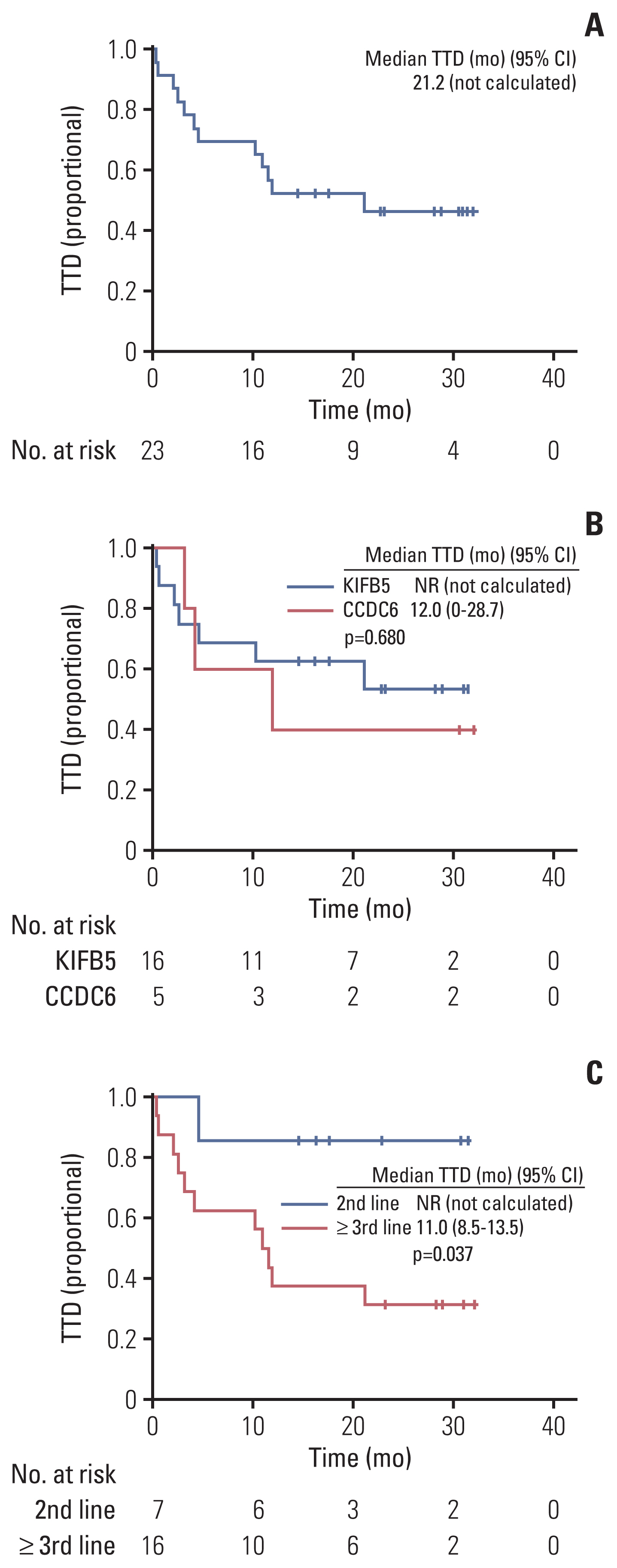
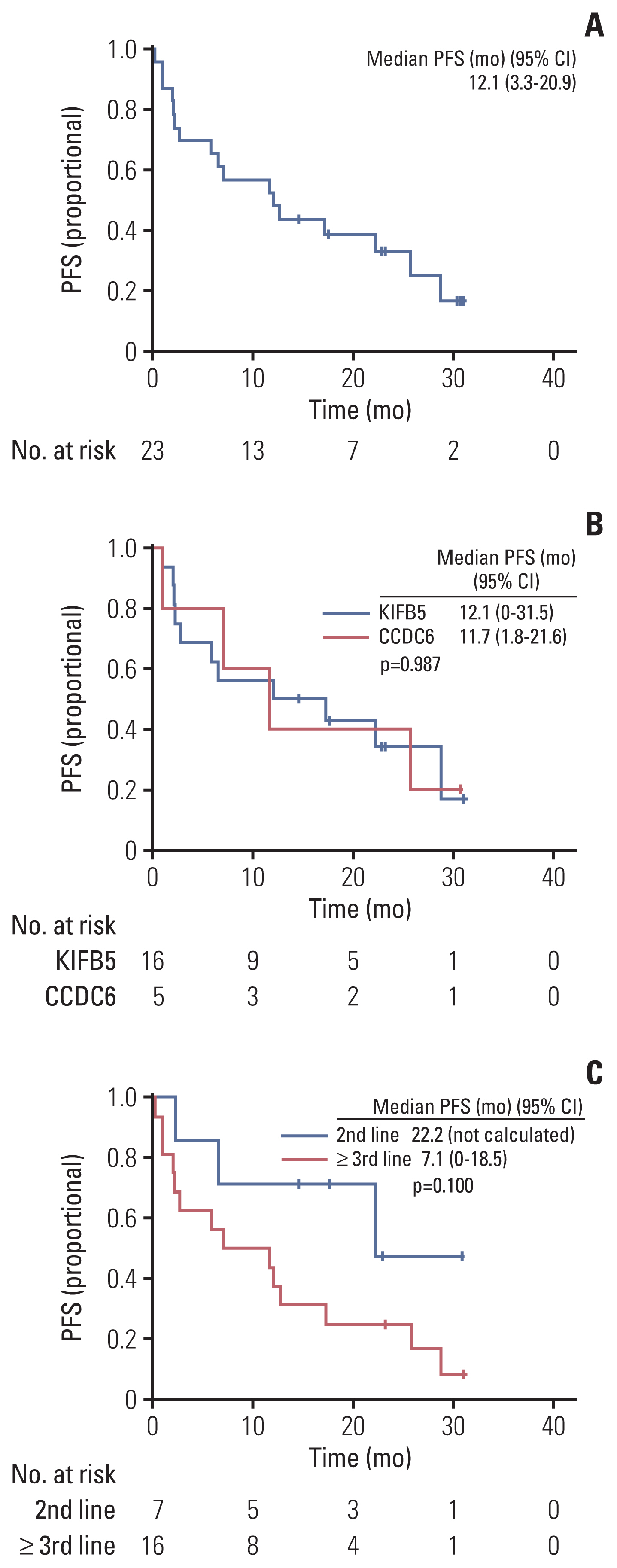
Between April 2020 and September 2021, 23 of 27 patients were enrolled in the EAP study. Two patients who were not analyzed due to brain metastasis and two patients whose expected survival was within 1 month were excluded from the analysis. After a median follow-up period of 15.6 months (95% confidence interval [CI], 10.0 to 21.2), ORR was 56.5%, the median PFS was 12.1 months (95% CI, 3.3 to 20.9), and the 12-month OS rate was 69.6%. The most frequent treatment-related adverse events (TRAEs) were edema (43.5%) and pneumonitis (39.1%). A total of 8.7% of patients experienced extra-pulmonary tuberculosis. TRAEs with a common grade of three or worse were neutropenia (43.5%) and anemia (34.8%). Dose reduction was required in nine patients (39.1%).
Conclusion
Pralsetinib presents a clinical benefit when used in patients with RET-rearranged NSCLC, consistent with a pivotal study.
Figure
Reference
-
References
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer;2020.2. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019; 94:1623–40.3. Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018; 36:911–9.4. Ali F, Neha K, Chauhan G. Pralsetinib: chemical and therapeutic development with FDA authorization for the management of RET fusion-positive non-small-cell lung cancers. Arch Pharm Res. 2022; 45:309–27.5. Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012; 18:378–81.6. Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. 2020; 38:1209–21.7. Lipson D, Capelletti M, Yelensky R, Otto G, Parker A, Jarosz M, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012; 18:382–4.8. Hegde A, Huang L, Liu S, Hess K, Cabanillas M, Hu M, et al. Responsiveness to immune checkpoint inhibitors in RET dependent cancers. Cancer Res. 2019; 79(13 Suppl):4997.9. Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021; 22:959–69.10. Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015; 16:e270–8.11. Lee YP, Jeong BH, Eun Y, Kang CI, Park S, Jung HA, et al. Extrapulmonary tuberculosis in patients with RET fusion-positive non-small cell lung cancer treated with pralsetinib: a Korean single-centre compassionate use experience. Eur J Cancer. 2021; 159:167–73.12. Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016; 17:1653–60.13. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017; 5:42–50.14. Velcheti V, Hida T, Reckamp KL, Yang JC, Nokihara H, Sachdev P, et al. Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion-positive adenocarcinoma of the lung. Ann Oncol. 2016; 27(Suppl 6):VI417.15. Drilon A, Oxnard G, Wirth L, Besse B, Gautschi O, Tan SW, et al. PL02. 08 Registrational results of LIBRETTO-001: a phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol. 2019; 14(10 Suppl):S6–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RET: A Multi-Faceted Gene in Human Cancer
- RET Fusion Genes in Korean Non-Small Cell Lung Cancer
- Overview of ALK and ROS1 Rearranged Lung Cancer
- Immunoglobulin and T-cell receptor gene rearrangement analysis in malignant lymphoid neoplasms
- EGF Induced RET Inhibitor Resistance in CCDC6-RET Lung Cancer Cells