Cancer Res Treat.
2023 Oct;55(4):1134-1143. 10.4143/crt.2023.311.
Targeting CD73 to Overcomes Resistance to First-Generation EGFR Tyrosine Kinase Inhibitors in Non–Small Cell Lung Cancer
- Affiliations
-
- 1Cancer Research Institute, Seoul National University, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Integrated Major in Innovative Medical Science, Seoul National University Graduate School, Seoul, Korea
- 4Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2547788
- DOI: http://doi.org/10.4143/crt.2023.311
Abstract
- Purpose
In patients with epidermal growth factor receptor (EGFR)-mutant non–small cell lung cancer (NSCLC), EGFR tyrosine kinase inhibitors (TKIs) improve response rate and survival. However, most patients eventually develop resistance. This study aimed to identify the role of CD73 in EGFR-mutant NSCLC and explore whether CD73 inhibition may serve as a therapeutic strategy in NSCLC patients with acquired resistance to EGFR-TKIs.
Materials and Methods
We evaluated the prognostic role of CD73 expression in EGFR-mutant NSCLC using tumor samples from a single institution. We silenced CD73 in EGFR-TKI–resistant cell lines using short hairpin RNA (shRNA) targeting CD73 and also transfected a vector alone as a negative control. Using these cell lines, cell proliferation and viability assays, immunoblot assays, cell cycle analysis, colony-forming assays, flow cytometry, and apoptosis analysis were performed.
Results
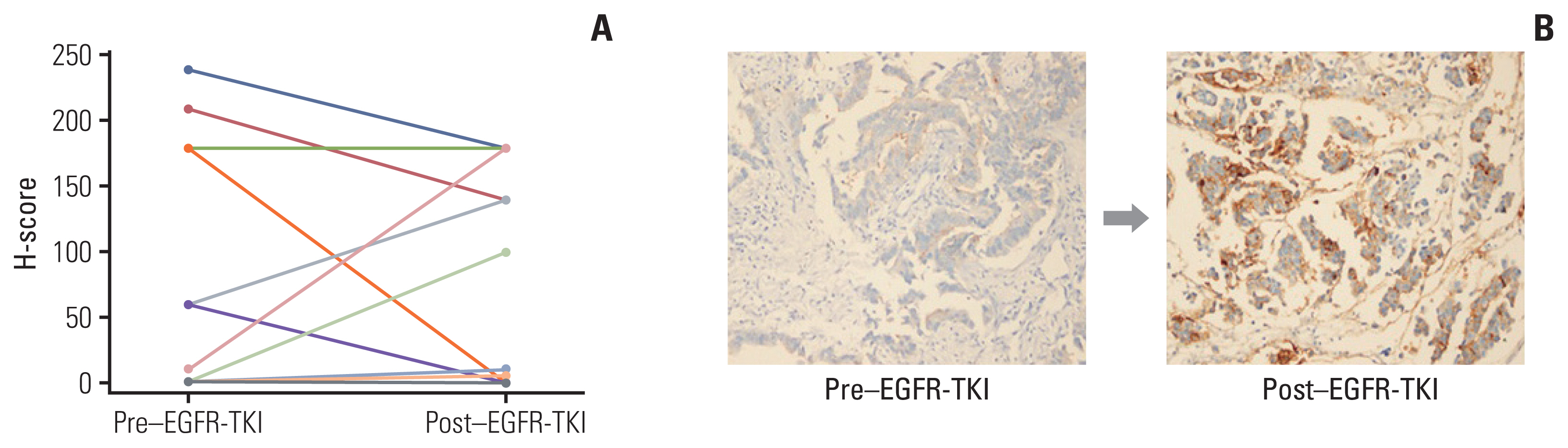
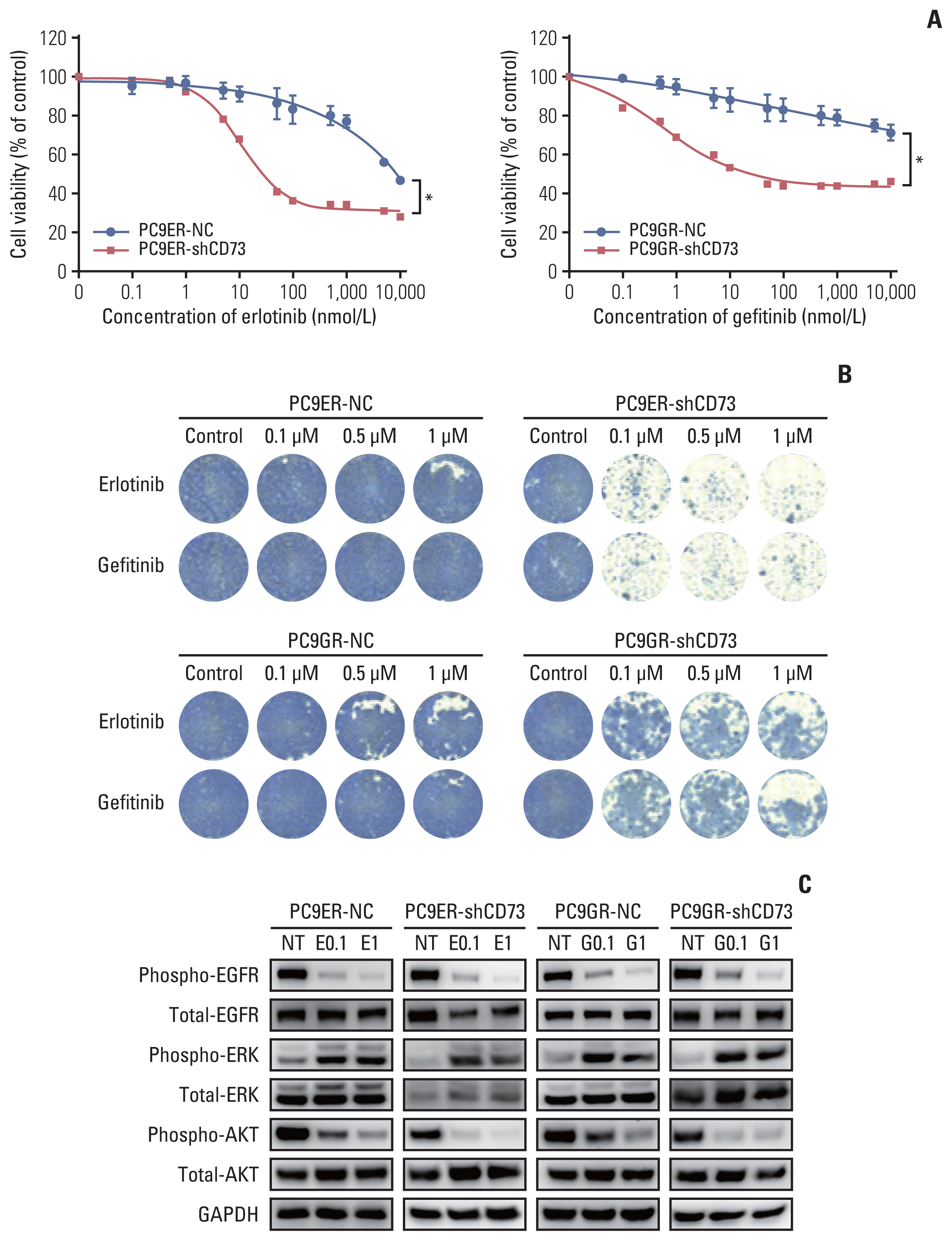
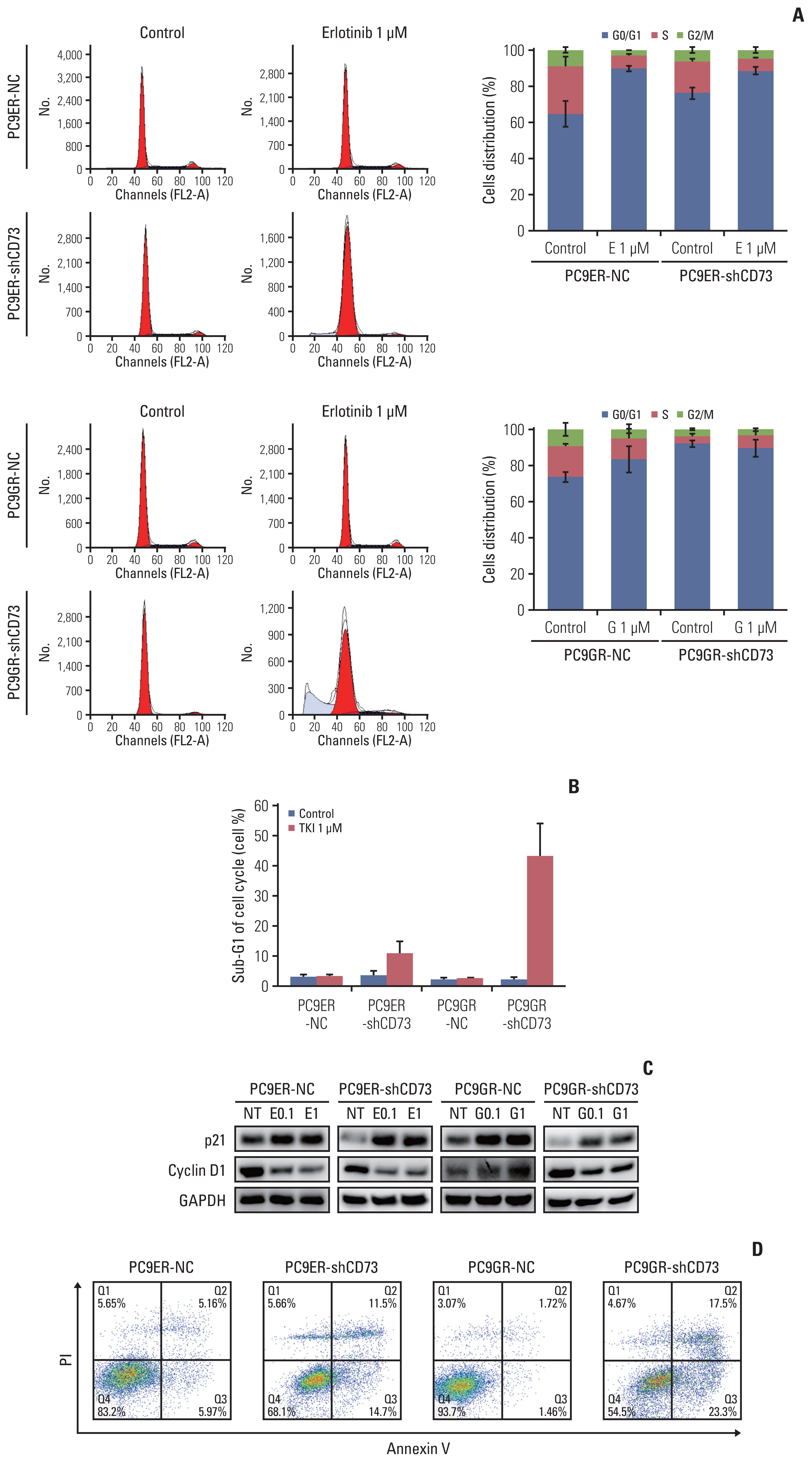
High expression of CD73 was associated with shorter survival in patients with metastatic EGFR-mutant NSCLC treated with first-generation EGFR-TKI. CD73 inhibition synergistically inhibited cell viability with first-generation EGFR-TKI treatment compared with the negative control. When CD73 inhibition and EGFR-TKI treatment were combined, G0/G1 cell cycle arrest was induced through the regulation of p21 and cyclin D1. In addition, the apoptosis rate was increased in CD73 shRNA-transfected cells treated with EGFR-TKI.
Conclusion
High expression of CD73 adversely affects the survival of patients with EGFR-mutant NSCLC. The study demonstrated that inhibiting CD73 in EGFR-TKI–resistant cell lines resulted in increased apoptosis and cell cycle arrest, which overcame the acquired resistance to first-generation EGFR-TKIs. Further research is needed to determine whether blocking CD73 plays a therapeutic role in EGFR-TKI–resistant patients with EGFR-mutant NSCLC.
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30.2. Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009; 10:432–3.3. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.4. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Onol. 2012; 13:239–46.5. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019; 17:1464–72.6. Park S, Keam B, Kim SH, Kim KH, Kim YJ, Kim JS, et al. Pemetrexed singlet versus nonpemetrexed-based platinum doublet as second-line chemotherapy after first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor failure in non-small cell lung cancer patients with EGFR mutations. Cancer Res Treat. 2015; 47:630–7.7. Yoshida T, Kuroda H, Oya Y, Shimizu J, Horio Y, Sakao Y, et al. Clinical outcomes of platinum-based chemotherapy according to T790M mutation status in EGFR-positive non-small cell lung cancer patients after initial EGFR-TKI failure. Lung Cancer. 2017; 109:89–91.8. Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001; 414:916–20.9. Ramkumar V, Hallam DM, Nie Z. Adenosine, oxidative stress and cytoprotection. Jpn J Pharmacol. 2001; 86:265–74.10. Shaikh G, Cronstein B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016; 12:191–7.11. Vijayan D, Young A, Teng MW, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017; 17:709–24.12. Jiang T, Xu X, Qiao M, Li X, Zhao C, Zhou F, et al. Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer. 2018; 18:267.13. Zhou L, Jia S, Chen Y, Wang W, Wu Z, Yu W, et al. The distinct role of CD73 in the progression of pancreatic cancer. J Mol Med (Berl). 2019; 97:803–15.14. Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010; 107:1547–52.15. Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019; 27:2411–25.16. Le X, Negrao MV, Reuben A, Federico L, Diao L, McGrail D, et al. Characterization of the immune landscape of EGFR-mutant NSCLC identifies CD73/adenosine pathway as a potential therapeutic target. J Thorac Oncol. 2021; 16:583–600.17. Gao ZW, Wang HP, Lin F, Wang X, Long M, Zhang HZ, et al. CD73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer. 2017; 17:135.18. Zhu J, Zeng Y, Li W, Qin H, Lei Z, Shen D, et al. CD73/NT5E is a target of miR-30a-5p and plays an important role in the pathogenesis of non-small cell lung cancer. Mol Cancer. 2017; 16:34.19. Xu Z, Gu C, Yao X, Guo W, Wang H, Lin T, et al. CD73 promotes tumor metastasis by modulating RICS/RhoA signaling and EMT in gastric cancer. Cell Death Dis. 2020; 11:202.20. Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, et al. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007; 24:439–48.21. Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim TM, et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol. 2015; 28:1154–66.22. Park HR, Ahn YO, Kim TM, Kim S, Kim S, Lee YS, et al. NK92-CD16 cells are cytotoxic to non-small cell lung cancer cell lines that have acquired resistance to tyrosine kinase inhibitors. Cytotherapy. 2019; 21:603–11.23. Inoue Y, Yoshimura K, Kurabe N, Kahyo T, Kawase A, Tanahashi M, et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget. 2017; 8:8738–51.24. Griesing S, Liao BC, Yang JC. CD73 is regulated by the EGFR-ERK signaling pathway in non-small cell lung cancer. Anticancer Res. 2021; 41:1231–42.25. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2020; 26:2037–46.26. Lupia M, Angiolini F, Bertalot G, Freddi S, Sachsenmeier KF, Chisci E, et al. CD73 regulates semness and epithelial-mesenchymal transition in ovarian cancer-initiating cells. Stem Cell Reports. 2018; 10:1412–25.27. Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014; 134:1466–73.28. Kim DW, Kim SW, Camidge DR, Rizvi NA, Marrone KA, Le X, et al. Abstract CT163: CD73 inhibitor oleclumab plus osimertinib for advanced EGFRm NSCLC: first report of a phase 1b/2 study. Cancer Res. 2021; 81(13_Suppl):CT163.29. Yu J, Wang X, Lu Q, Wang J, Li L, Liao X, et al. Extracellular 5′-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3beta/beta-catenin/cyclinD1 signaling pathway. Int J Cancer. 2018; 142:959–67.30. Zhi X, Wang Y, Zhou X, Yu J, Jian R, Tang S, et al. RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci. 2010; 101:2561–9.31. Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, Jose V, et al. CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 2017; 77:5652–63.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EGFR C797S as a Resistance Mechanism of Lazertinib in Non-small Cell Lung Cancer with EGFR T790M Mutation
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Chronicles of EGFR Tyrosine Kinase Inhibitors: Targeting EGFR C797S Containing Triple Mutations
- EGFR Tyrosine Kinase Inhibitors for NSCLC
- Mechanisms of Acquired Resistance to Epidermal Growth Factor Receptor Inhibitors and Overcoming Strategies in Lung Cancer