Endocrinol Metab.
2023 Oct;38(5):463-471. 10.3803/EnM.2023.1820.
Multiomics Approach to Acromegaly: Unveiling Translational Insights for Precision Medicine
- Affiliations
-
- 1Endocrinology, Institute of Endocrine Research, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2546977
- DOI: http://doi.org/10.3803/EnM.2023.1820
Abstract
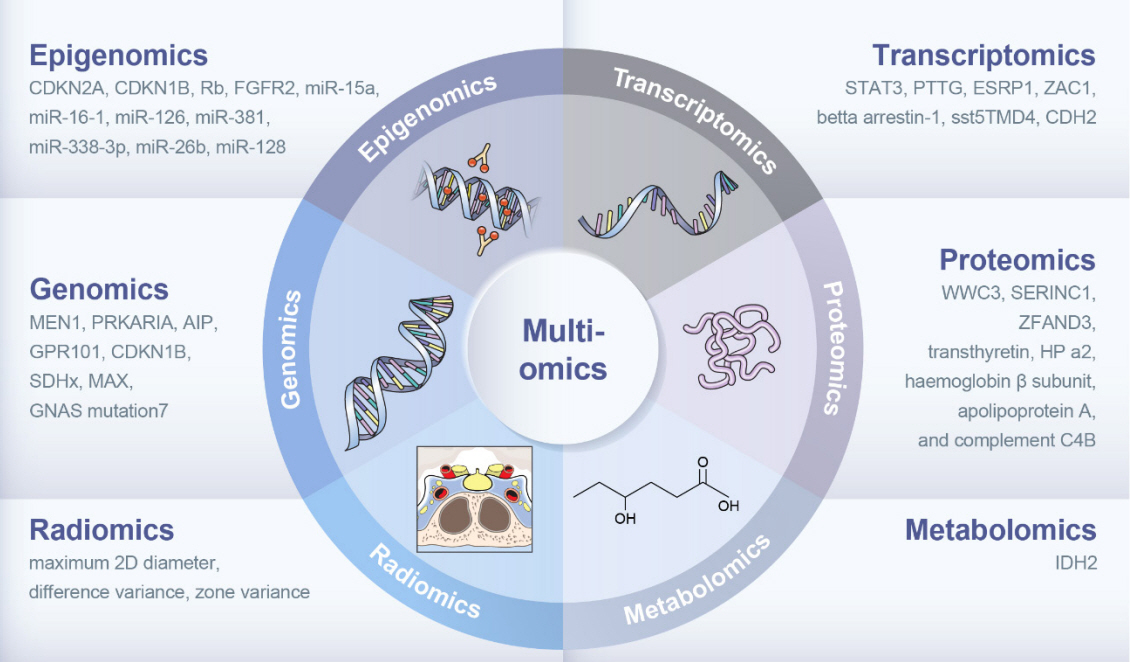
- The clinical characteristics and prognoses of acromegaly vary among patients. Assessment of current and novel predictors can lead to multilevel categorization of patients, allowing integration into new clinical guidelines and a reduction in the increased morbidity and mortality associated with acromegaly. Despite advances in the diagnosis and treatment of acromegaly, its pathophysiology remains unclear. Recent advancements in multiomics technologies, including genomics, transcriptomics, proteomics, metabolomics, and radiomics, have offered new opportunities to unravel the complex pathophysiology of acromegaly. This review comprehensively explores the emerging role of multiomics approaches in elucidating the molecular landscape of acromegaly. We discuss the potential implications of multiomics data integration in the development of novel diagnostic tools, identification of therapeutic targets, and the prospects of precision medicine in acromegaly management. By integrating diverse omics datasets, these approaches can provide valuable insights into disease mechanisms, facilitate the identification of diagnostic biomarkers, and identify potential therapeutic targets for precision medicine in the management of acromegaly.
Figure
Reference
-
1. Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J. Pituitary tumours: acromegaly. Best Pract Res Clin Endocrinol Metab. 2009; 23:555–74.2. Kim J, Hong N, Choi J, Moon JH, Kim EH, Hong JW, et al. Sex differences in mortality in patients with acromegaly: a nationwide cohort study in Korea. Eur J Endocrinol. 2023; 189:225–34.
Article3. Park KH, Lee EJ, Seo GH, Ku CR. Risk for acromegaly-related comorbidities by sex in Korean acromegaly. J Clin Endocrinol Metab. 2020; 105:dgz317.
Article4. Gadelha MR, Kasuki L, Lim DS, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. 2019; 40:268–332.
Article5. Hwang YA, Lee HW, Ahn SH, Lee EJ, Ku CR, Kim SU. Positive association between nonalcoholic fatty liver disease and growth hormone deficiency in patients with nonfunctioning pituitary adenoma. Front Endocrinol (Lausanne). 2023; 13:1057769.
Article6. Ribeiro-Oliveira A Jr, Barkan A. The changing face of acromegaly: advances in diagnosis and treatment. Nat Rev Endocrinol. 2012; 8:605–11.7. Langlois F, McCartney S, Fleseriu M. Recent progress in the medical therapy of pituitary tumors. Endocrinol Metab (Seoul). 2017; 32:162–70.
Article8. Ku CR, Kim EH, Oh MC, Lee EJ, Kim SH. Surgical and endocrinological outcomes in the treatment of growth hormonesecreting pituitary adenomas according to the shift of surgical paradigm. Neurosurgery. 2012; 71(2 Suppl Operative):ons 192–203.
Article9. Jung IH, Choi S, Ku CR, Lee SG, Lee EJ, Kim SH, et al. Revisiting the role of insulin-like growth factor-1 measurement after surgical treatment of acromegaly. J Clin Endocrinol Metab. 2021; 106:e2589–99.
Article10. Kim J, Hwang YA, Park YW, Moon JH, Kim EH, Hong JW, et al. Revisiting growth hormone nadir cut-offs for remission in patients with acromegaly. Eur J Endocrinol. 2022; 186:657–65.
Article11. Park SH, Ku CR, Moon JH, Kim EH, Kim SH, Lee EJ. Age- and sex-specific differences as predictors of surgical remission among patients with acromegaly. J Clin Endocrinol Metab. 2018; 103:909–16.
Article12. Kim EH, Oh MC, Lee EJ, Kim SH. Predicting long-term remission by measuring immediate postoperative growth hormone levels and oral glucose tolerance test in acromegaly. Neurosurgery. 2012; 70:1106–13.
Article13. Yan J, Risacher SL, Shen L, Saykin AJ. Network approaches to systems biology analysis of complex disease: integrative methods for multi-omics data. Brief Bioinform. 2018; 19:1370–81.
Article14. Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017; 18:83.
Article15. Ku CR, Melnikov V, Zhang Z, Lee EJ. Precision therapy in acromegaly caused by pituitary tumors: how close is it to reality? Endocrinol Metab (Seoul). 2020; 35:206–16.
Article16. Burke W, Psaty BM. Personalized medicine in the era of genomics. JAMA. 2007; 298:1682–4.
Article17. Uffelmann E, Huang QQ, Munung NS, De Vries J, Okada Y, Martin AR, et al. Genome-wide association studies. Nat Rev Methods Primers. 2021; 1:59.
Article18. Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014; 371:2363–74.
Article19. Gadelha MR, Kasuki L, Korbonits M. The genetic background of acromegaly. Pituitary. 2017; 20:10–21.
Article20. Caimari F, Korbonits M. Novel genetic causes of pituitary adenomas. Clin Cancer Res. 2016; 22:5030–42.
Article21. Gillam MP, Ku CR, Lee YJ, Kim J, Kim SH, Lee SJ, et al. Somatotroph-specific Aip-deficient mice display pretumorigenic alterations in cell-cycle signaling. J Endocr Soc. 2017; 1:78–95.
Article22. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989; 340:692–6.
Article23. Landis CA, Harsh G, Lyons J, Davis RL, McCormick F, Bourne HR. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990; 71:1416–20.24. Jung H, Kim K, Kim D, Moon JH, Kim EH, Kim SH, et al. Associations of GNAS mutations with surgical outcomes in patients with growth hormone-secreting pituitary adenoma. Endocrinol Metab (Seoul). 2021; 36:342–50.
Article25. Ye Z, Li Z, Wang Y, Mao Y, Shen M, Zhang Q, et al. Common variants at 10p12.31, 10q21.1 and 13q12.13 are associated with sporadic pituitary adenoma. Nat Genet. 2015; 47:793–7.
Article26. Lecoq AL, Kamenicky P, Guiochon-Mantel A, Chanson P. Genetic mutations in sporadic pituitary adenomas: what to screen for? Nat Rev Endocrinol. 2015; 11:43–54.
Article27. Hage M, Viengchareun S, Brunet E, Villa C, Pineau D, Bouligand J, et al. Genomic alterations and complex subclonal architecture in sporadic GH-secreting pituitary adenomas. J Clin Endocrinol Metab. 2018; 103:1929–39.
Article28. Bi WL, Greenwald NF, Ramkissoon SH, Abedalthagafi M, Coy SM, Ligon KL, et al. Clinical identification of oncogenic drivers and copy-number alterations in pituitary tumors. Endocrinology. 2017; 158:2284–91.
Article29. Yoshino A, Katayama Y, Ogino A, Watanabe T, Yachi K, Ohta T, et al. Promoter hypermethylation profile of cell cycle regulator genes in pituitary adenomas. J Neurooncol. 2007; 83:153–62.
Article30. Kirsch M, Morz M, Pinzer T, Schackert HK, Schackert G. Frequent loss of the CDKN2C (p18INK4c) gene product in pituitary adenomas. Genes Chromosomes Cancer. 2009; 48:143–54.31. Hossain MG, Iwata T, Mizusawa N, Qian ZR, Shima SW, Okutsu T, et al. Expression of p18(INK4C) is down-regulated in human pituitary adenomas. Endocr Pathol. 2009; 20:114–21.
Article32. Ling C, Pease M, Shi L, Punj V, Shiroishi MS, Commins D, et al. A pilot genome-scale profiling of DNA methylation in sporadic pituitary macroadenomas: association with tumor invasion and histopathological subtype. PLoS One. 2014; 9:e96178.
Article33. Yacqub-Usman K, Duong CV, Clayton RN, Farrell WE. Preincubation of pituitary tumor cells with the epidrugs zebularine and trichostatin A are permissive for retinoic acid-augmented expression of the BMP-4 and D2R genes. Endocrinology. 2013; 154:1711–21.
Article34. Li T, Huang H, Huang B, Huang B, Lu J. Histone acetyltransferase p300 regulates the expression of human pituitary tumor transforming gene (hPTTG). J Genet Genomics. 2009; 36:335–42.
Article35. Grande IP, Amorim PV, Freire AC, Jallad RS, Musolino NR, Cescato VA, et al. Differential gene expression of sirtuins between somatotropinomas and nonfunctioning pituitary adenomas. Pituitary. 2018; 21:355–61.
Article36. DeVore SB, Young CH, Li G, Sundararajan A, Ramaraj T, Mudge J, et al. Histone citrullination represses microRNA expression, resulting in increased oncogene mRNAs in somatolactotrope cells. Mol Cell Biol. 2018; 38:e00084–18.
Article37. Zhang P, Wu W, Chen Q, Chen M. Non-coding RNAs and their integrated networks. J Integr Bioinform. 2019; 16:20190027.
Article38. Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012; 482:339–46.
Article39. Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol. 2005; 204:280–5.
Article40. Elzein S, Goodyer CG. Regulation of human growth hormone receptor expression by microRNAs. Mol Endocrinol. 2014; 28:1448–59.
Article41. D’Angelo D, Palmieri D, Mussnich P, Roche M, Wierinckx A, Raverot G, et al. Altered microRNA expression profile in human pituitary GH adenomas: down-regulation of miRNA targeting HMGA1, HMGA2, and E2F1. J Clin Endocrinol Metab. 2012; 97:E1128–38.42. Mao ZG, He DS, Zhou J, Yao B, Xiao WW, Chen CH, et al. Differential expression of microRNAs in GH-secreting pituitary adenomas. Diagn Pathol. 2010; 5:79.
Article43. Lee YJ, Cho JM, Moon JH, Ku CR, Kim J, Kim SH, et al. Increased miR-338-3p expression correlates with invasiveness of GH-producing pituitary adenomas. Endocrine. 2017; 58:184–9.
Article44. Salehi F, Kovacs K, Scheithauer BW, Lloyd RV, Cusimano M. Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocr Relat Cancer. 2008; 15:721–43.
Article45. Palumbo T, Faucz FR, Azevedo M, Xekouki P, Iliopoulos D, Stratakis CA. Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTENAKT pathway. Oncogene. 2013; 32:1651–9.
Article46. Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020; 47:4587–629.
Article47. Xie P, Han Q, Liu D, Yao D, Lu X, Wang Z, et al. miR-525-5p modulates proliferation and epithelial-mesenchymal transition of glioma by targeting Stat-1. Onco Targets Ther. 2020; 13:9957–66.48. Gupta G, Chellappan DK, de Jesus Andreoli Pinto T, Hansbro PM, Bebawy M, Dua K. Tumor suppressor role of miR-503. Panminerva Med. 2018; 60:17–24.
Article49. Denes J, Kasuki L, Trivellin G, Colli LM, Takiya CM, Stiles CE, et al. Regulation of aryl hydrocarbon receptor interacting protein (AIP) protein expression by MiR-34a in sporadic somatotropinomas. PLoS One. 2015; 10:e0117107.
Article50. Bogner EM, Daly AF, Gulde S, Karhu A, Irmler M, Beckers J, et al. miR-34a is upregulated in AIP-mutated somatotropinomas and promotes octreotide resistance. Int J Cancer. 2020; 147:3523–38.51. Fan X, Mao Z, He D, Liao C, Jiang X, Lei N, et al. Expression of somatostatin receptor subtype 2 in growth hormone-secreting pituitary adenoma and the regulation of miR-185. J Endocrinol Invest. 2015; 38:1117–28.
Article52. Hegde PS, White IR, Debouck C. Interplay of transcriptomics and proteomics. Curr Opin Biotechnol. 2003; 14:647–51.
Article53. Zhou C, Jiao Y, Wang R, Ren SG, Wawrowsky K, Melmed S. STAT3 upregulation in pituitary somatotroph adenomas induces growth hormone hypersecretion. J Clin Invest. 2015; 125:1692–702.
Article54. Bernal JA, Luna R, Espina A, Lazaro I, Ramos-Morales F, Romero F, et al. Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nat Genet. 2002; 32:306–11.
Article55. Chesnokova V, Zonis S, Kovacs K, Ben-Shlomo A, Wawrowsky K, Bannykh S, et al. p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci U S A. 2008; 105:17498–503.56. Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, et al. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf). 2006; 65:536–43.
Article57. Lekva T, Berg JP, Fougner SL, Olstad OK, Ueland T, Bollerslev J. Gene expression profiling identifies ESRP1 as a potential regulator of epithelial mesenchymal transition in somatotroph adenomas from a large cohort of patients with acromegaly. J Clin Endocrinol Metab. 2012; 97:E1506–14.
Article58. Gatto F, Biermasz NR, Feelders RA, Kros JM, Dogan F, van der Lely AJ, et al. Low beta-arrestin expression correlates with the responsiveness to long-term somatostatin analog treatment in acromegaly. Eur J Endocrinol. 2016; 174:651–62.
Article59. Theodoropoulou M, Zhang J, Laupheimer S, Paez-Pereda M, Erneux C, Florio T, et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006; 66:1576–82.
Article60. Chahal HS, Trivellin G, Leontiou CA, Alband N, Fowkes RC, Tahir A, et al. Somatostatin analogs modulate AIP in somatotroph adenomas: the role of the ZAC1 pathway. J Clin Endocrinol Metab. 2012; 97:E1411–20.
Article61. Duran-Prado M, Gahete MD, Martinez-Fuentes AJ, Luque RM, Quintero A, Webb SM, et al. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab. 2009; 94:2634–43.
Article62. Luque RM, Ibanez-Costa A, Neto LV, Taboada GF, Hormaechea-Agulla D, Kasuki L, et al. Truncated somatostatin receptor variant sst5TMD4 confers aggressive features (proliferation, invasion and reduced octreotide response) to somatotropinomas. Cancer Lett. 2015; 359:299–306.
Article63. Ben-Shlomo A, Deng N, Ding E, Yamamoto M, Mamelak A, Chesnokova V, et al. DNA damage and growth hormone hypersecretion in pituitary somatotroph adenomas. J Clin Invest. 2020; 130:5738–55.
Article64. Ronchi CL, Peverelli E, Herterich S, Weigand I, Mantovani G, Schwarzmayr T, et al. Landscape of somatic mutations in sporadic GH-secreting pituitary adenomas. Eur J Endocrinol. 2016; 174:363–72.
Article65. Ku CR, Lim H, Lee YJ, Kim SH, Kim D, Kim SH, et al. Novel somatic variants involved in biochemical activity of pure growth hormone-secreting pituitary adenoma without GNAS variant. Sci Rep. 2021; 11:16530.
Article66. Lyu L, Jiang Y, Ma W, Li H, Liu X, Li L, et al. Single-cell sequencing of PIT1-positive pituitary adenoma highlights the pro-tumour microenvironment mediated by IFN-γ-induced tumour-associated fibroblasts remodelling. Br J Cancer. 2023; 128:1117–33.
Article67. Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: technologies and their applications. J Chromatogr Sci. 2017; 55:182–96.
Article68. Zhang Z, Wu S, Stenoien DL, Pasa-Tolic L. High-throughput proteomics. Annu Rev Anal Chem (Palo Alto Calif). 2014; 7:427–54.
Article69. Yamato A, Nagano H, Gao Y, Matsuda T, Hashimoto N, Nakayama A, et al. Proteogenomic landscape and clinical characterization of GH-producing pituitary adenomas/somatotroph pituitary neuroendocrine tumors. Commun Biol. 2022; 5:1304.
Article70. Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, Jorgensen JO, Kopchick JJ. Serum proteome changes in acromegalic patients following transsphenoidal surgery: novel biomarkers of disease activity. Eur J Endocrinol. 2011; 164:157–67.
Article71. Feng J, Gao H, Zhang Q, Zhou Y, Li C, Zhao S, et al. Metabolic profiling reveals distinct metabolic alterations in different subtypes of pituitary adenomas and confers therapeutic targets. J Transl Med. 2019; 17:291.
Article72. Oklu R, Deipolyi AR, Wicky S, Ergul E, Deik AA, Chen JW, et al. Identification of small compound biomarkers of pituitary adenoma: a bilateral inferior petrosal sinus sampling study. J Neurointerv Surg. 2014; 6:541–6.
Article73. Feng J, Zhang Q, Zhou Y, Yu S, Hong L, Zhao S, et al. Integration of proteomics and metabolomics revealed metabolite-protein networks in ACTH-secreting pituitary adenoma. Front Endocrinol (Lausanne). 2018; 9:678.
Article74. Ijare OB, Holan C, Hebert J, Sharpe MA, Baskin DS, Pichumani K. Elevated levels of circulating betahydroxybutyrate in pituitary tumor patients may differentiate prolactinomas from other immunohistochemical subtypes. Sci Rep. 2020; 10:1334.
Article75. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016; 278:563–77.
Article76. Park YW, Choi YS, Ahn SS, Chang JH, Kim SH, Lee SK. Radiomics MRI phenotyping with machine learning to predict the grade of lower-grade gliomas: a study focused on nonenhancing tumors. Korean J Radiol. 2019; 20:1381–9.
Article77. Park HH, Kim EH, Ku CR, Lee EJ, Kim SH. Outcomes of aggressive surgical resection in growth hormone-secreting pituitary adenomas with cavernous sinus invasion. World Neurosurg. 2018; 117:e280–9.
Article78. Kim EH, Oh MC, Chang JH, Moon JH, Ku CR, Chang WS, et al. Postoperative gamma knife radiosurgery for cavernous sinus-invading growth hormone-secreting pituitary adenomas. World Neurosurg. 2018; 110:e534–45.
Article79. Park YW, Kang Y, Ahn SS, Ku CR, Kim EH, Kim SH, et al. Radiomics model predicts granulation pattern in growth hormone-secreting pituitary adenomas. Pituitary. 2020; 23:691–700.
Article80. Park YW, Eom J, Kim S, Kim H, Ahn SS, Ku CR, et al. Radiomics with ensemble machine learning predicts dopamine agonist response in patients with prolactinoma. J Clin Endocrinol Metab. 2021; 106:e3069–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precision Medicine in Treatment: Based on Multiomics or Clinical Data
- Precision Therapy in Acromegaly Caused by Pituitary Tumors: How Close Is It to Reality?
- Long-Term Prognosis and Systemic Impact of Acromegaly: Analyses Utilizing Korean National Health Insurance Data
- Medical Treatment of Acromegaly
- Precision Medicine in Type 2 Diabetes