J Liver Cancer.
2023 Sep;23(2):350-361. 10.17998/jlc.2023.08.10.
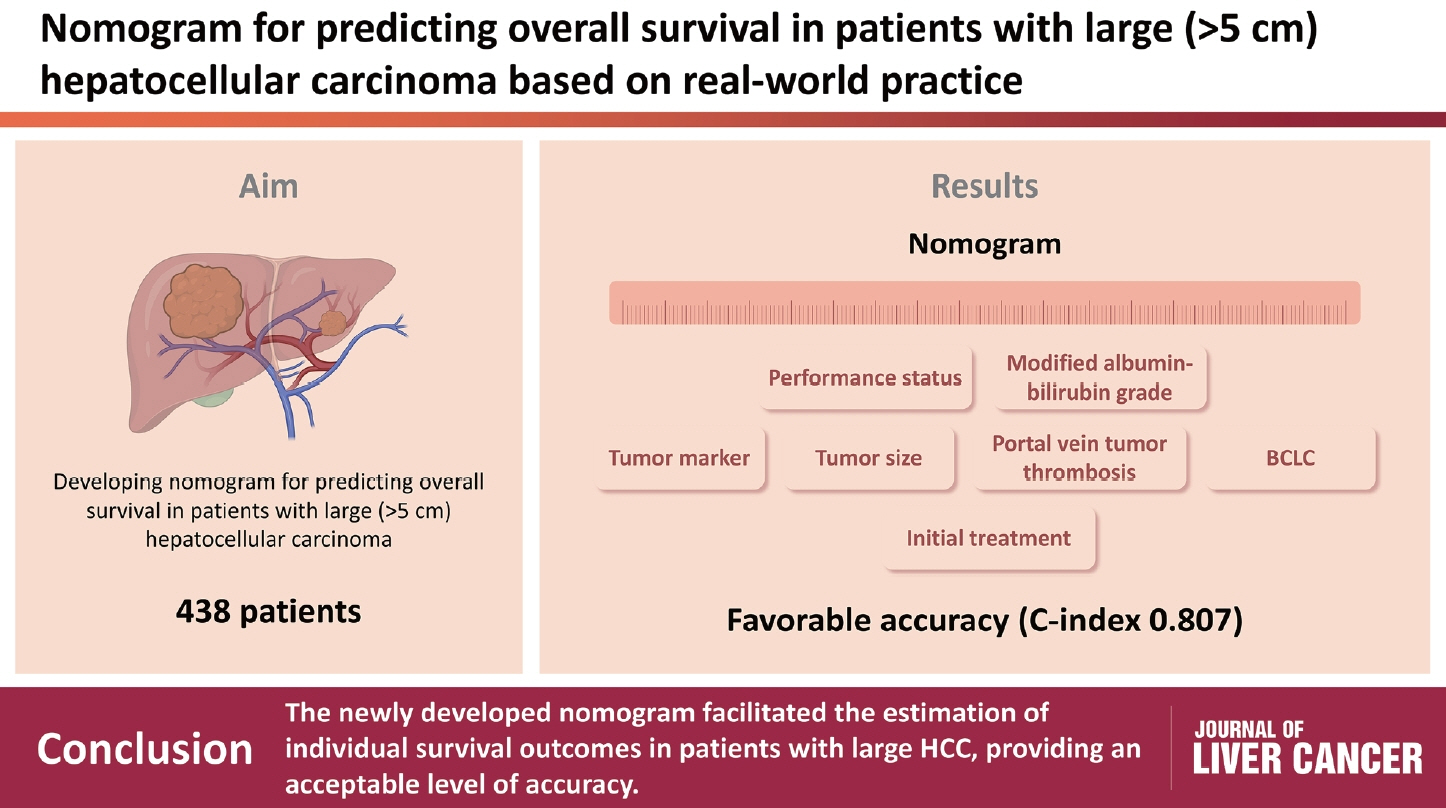
Nomogram for predicting overall survival in patients with large (>5 cm) hepatocellular carcinoma based on real-world practice
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Intenal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2546415
- DOI: http://doi.org/10.17998/jlc.2023.08.10
Abstract
- Background
/Aim: Patients with large (>5 cm) hepatocellular carcinoma (HCC) have limited treatment options, thus necessitating the identification of prognostic factors and the development of predictive tools. This study aimed to identify prognostic factors and to construct a nomogram to predict survival outcomes in patients with large HCC.
Methods
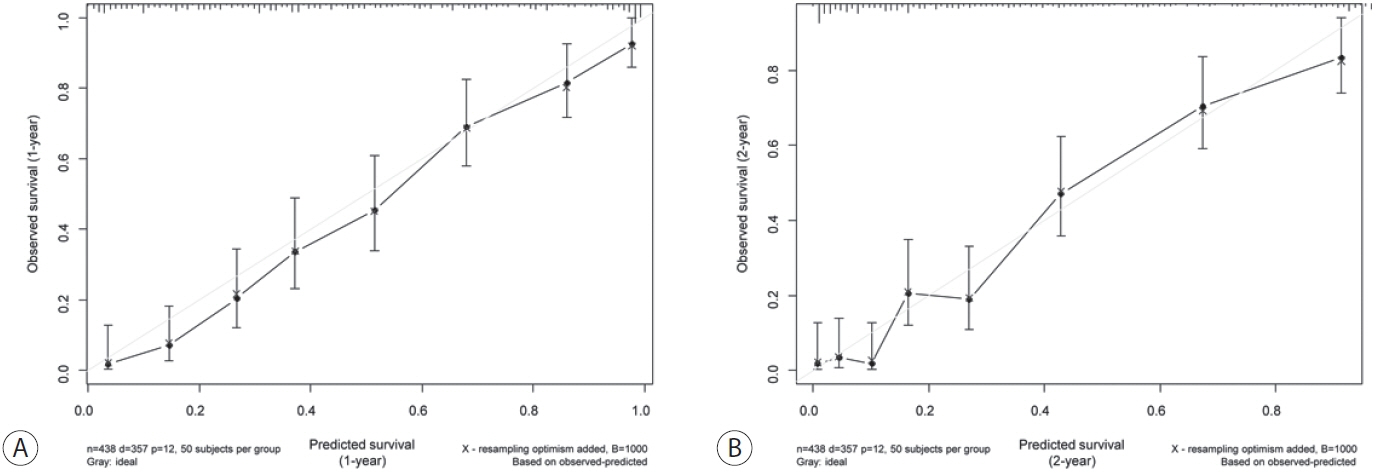
A cohort of 438 patients, who were diagnosed with large HCC at a tertiary hospital between 2015 and 2018, was analyzed. Cox proportional hazards models were used to identify key prognosticators of overall survival (OS), and an independent set of prognostic factors was used to develop a nomogram. The discrimination and calibration abilities of the nomogram were assessed and internal validation was performed using cross-validation and bootstrapping methods.
Results
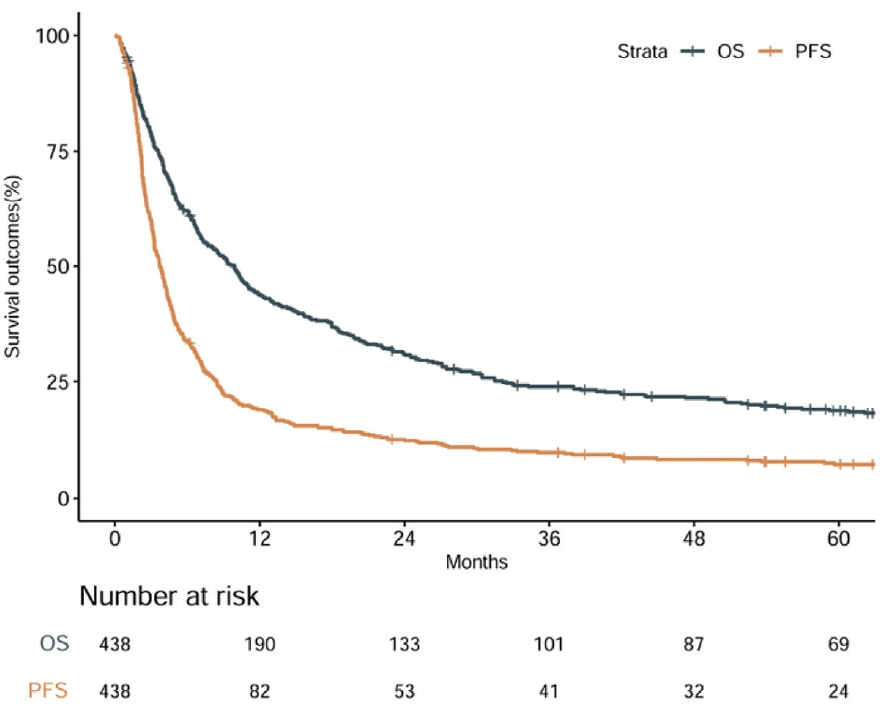
During a median follow-up of 9.3 months, the median OS was 9.9 months, and the 1-year OS rate was 43.9%. Multivariable Cox regression analysis revealed that performance status, modified albumin-bilirubin grade, tumor size, extent of portal vein tumor thrombosis, and initial treatment significantly affected OS. The newly developed nomogram incorporating these variables demonstrated favorable accuracy (Harrell’s concordance index, 0.807).
Conclusions
The newly developed nomogram facilitated the estimation of individual survival outcomes in patients with large HCC, providing an acceptable level of accuracy.
Keyword
Figure
Reference
-
References
1. Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N, et al. Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol. 2015; 7:2274–2291.2. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245–1255.3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.4. Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002; 194:592–602.5. Chen XP, Qiu FZ, Wu ZD, Zhang BX. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol. 2006; 12:4652–4655.6. Nagasue N, Kohno H, Chang YC, Taniura H, Yamanoi A, Uchida M, et al. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg. 1993; 217:375–384.7. Chon YE, Lee HA, Yoon JS, Park JY, Kim BH, Lee IJ, et al. Hepatocellular carcinoma in Korea between 2012 and 2014: an analysis of data from the Korean Nationwide Cancer Registry. J Liver Cancer. 2020; 20:135–147.8. Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res. 2018; 10:4401–4410.9. Hong SK, Lee KW, Lee S, Hong SY, Suh S, Han ES, et al. Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis. Ann Surg Treat Res. 2022; 102:193–204.10. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008; 26:1364–1370.11. Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019; 14:e0210730.12. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015; 33:765–770.13. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.14. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33:550–558.15. Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017; 6:325–336.16. Kudo M. Newly developed modified albi grade shows better prognostic and predictive value for hepatocellular carcinoma. Liver Cancer. 2021; 11:1–8.17. Yamashita Y, Taketomi A, Shirabe K, Aishima S, Tsuijita E, Morita K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (≥ 10 cm in diameter). J Surg Oncol. 2011; 104:292–298.18. Lim C, Compagnon P, Sebagh M, Salloum C, Calderaro J, Luciani A, et al. Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB (Oxford). 2015; 17:611–623.19. Bogdanovic A, Bulajic P, Masulovic D, Bidzic N, Zivanovic M, Galun D. Liver resection versus transarterial chemoembolization for huge hepatocellular carcinoma: a propensity score matched analysis. Sci Rep. 2021; 11:4493.20. Min YW, Lee JH, Gwak GY, Paik YH, Lee JH, Rhee PL, et al. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 2014; 29:1043–1048.21. Zhu SL, Zhong JH, Ke Y, Ma L, You XM, Li LQ. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol. 2015; 21:9630–9637.22. Wei CY, Chen PC, Chau GY, Lee RC, Chen PH, Huo TI, et al. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann Transl Med. 2020; 8:238.23. Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018; 287:705–714.24. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011; 47:2117–2127.25. Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019; 70:684–691.26. Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2020; 107:106–115.27. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.28. Li H, Wu Z, Chen J, Su K, Guo L, Xu K, et al. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med. 2022; Dec. 10. doi: 10.1007/s10238-022-00972-4. [Epub ahead of print].29. Yang M, Yuan JQ, Bai M, Han GH. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014; 41:6575–6582.30. Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, et al. Albumin-Bilirubin (ALBI) grade as part of the evidencebased clinical practice guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and Child-Pugh classifications. Liver Cancer. 2017; 6:204–215.31. Fang KC, Kao WY, Su CW, Chen PC, Lee PC, Huang YH, et al. The prognosis of single large hepatocellular carcinoma was distinct from Barcelona Clinic Liver Cancer stage A or B: the role of albumin-bilirubin grade. Liver Cancer. 2018; 7:335–358.32. Lee DS, Seong J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer. 2014; 3:18–30.33. Choe WH. Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus? J Liver Cancer. 2022; 22:1–3.34. Zhang F, Lu CD, Zhang XP, Chen ZH, Zhong CQ, Hu YR, et al. The impact of portal vein tumor thrombus on long-term survival after liver resection for primary hepatic malignancy. HPB (Oxford). 2020; 22:1025–1033.35. Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, et al. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001; 7:28–32.36. Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012; 82:2004–2011.37. Choi Y, Kim JW, Cha H, Han KH, Seong J. Overall response of both intrahepatic tumor and portal vein tumor thrombosis is a good prognostic factor for hepatocellular carcinoma patients receiving concurrent chemoradiotherapy. J Radiat Res. 2014; 55:113–120.38. Mähringer-Kunz A, Steinle V, Düber C, Weinmann A, Koch S, Schmidtmann I, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: the more, the worse? Liver Int. 2019; 39:324–331.39. Li JH, Yin X, Fan WS, Zhang L, Chen RX, Chen Y, et al. Development of a prognostic scoring system for hepatocellular carcinoma patients with main portal vein tumor thrombus undergoing conventional transarterial chemoembolization: an analysis of 173 patients. Front Oncol. 2021; 11:671171.40. Han K, Kim JH, Ko GY, Gwon DI, Sung KB. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol. 2016; 22:407–416.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nomogram for Predicting Survival for Oral Squamous Cell Carcinoma
- Clinical practice guidelines and real-world practice for hepatocellular carcinoma in Taiwan: Bridging the gap
- Nomograms to Predict the Individual Survival of Patients with Solitary Hepatocellular Carcinoma after Hepatectomy
- Prognostic Factors and Clinicopathologic Features after Resection of Small Hepatocellular Carcinoma (< or =2 cm)
- Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma and Extrahepatic Metastasis