J Korean Neurosurg Soc.
2023 Sep;66(5):536-542. 10.3340/jkns.2023.0024.
Intrawound Vancomycin Powder Application for Preventing Surgical Site Infection Following Cranioplasty
- Affiliations
-
- 1Department of Neurosurgery, Bundang Jesaeng General Hospital, Daejin Medical Center, Seongnam, Korea
- 2Department of Neurosurgery, Myongji St Mary’s Hospital, Seoul, Korea
- 3Department of Neurosurgery, Anyang Sam Hospital, Anyang, Korea
- 4Department of Neurosurgery, Jeju National University Hospital, Jeju, Korea
- 5Department of Neurosurgery, Bundang CHA Medical Center, CHA University, Seongnam, Korea
- KMID: 2545344
- DOI: http://doi.org/10.3340/jkns.2023.0024
Abstract
Objective
: Surgical site infection is the most detrimental complication following cranioplasty. In other surgical fields, intrawound vancomycin powder application has been introduced to prevent surgical site infection and is widely used based on results in multiple studies. This study evaluated the effect of intrawound vancomycin powder in cranioplasty compared with the conventional method without topical antibiotics.
Methods
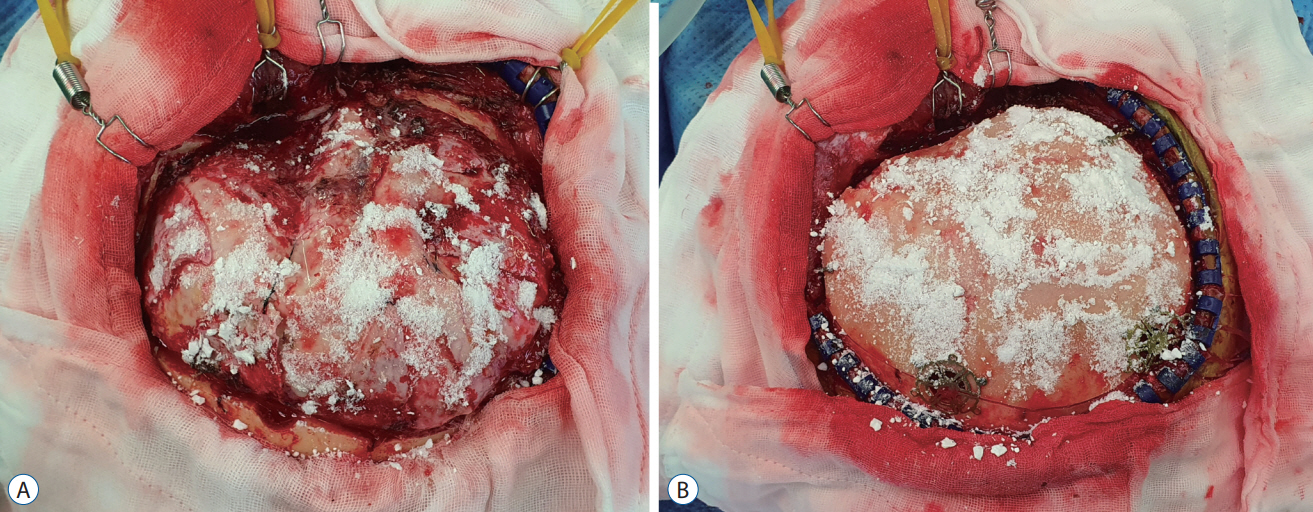
: This retrospective study included 580 patients with skull defects who underwent cranioplasty between August 1, 1998 and December 31, 2021. The conventional method was used in 475 (81.9%; conventional group) and vancomycin powder (1 g) was applied on the dura mater and bone flap in 105 patients (18.1%; vancomycin powder group). Surgical site infection was defined as infection of the incision, organ, or space that occurred after cranioplasty. Surgical site infection within 1-year surveillance period was compared between the conventional and vancomycin powder groups with logistic regression analysis. Penalized likelihood estimation method was used in logistic regression to deal with zero events. All local and systemic adverse events associated with topical vancomycin application were also evaluated.
Results
: Surgical site infection occurred in 31 patients (5.3%) and all were observed in the conventional group. The median time between cranioplasty and detection of surgical site infection was 13 days (range, 4–333). Staphylococci were the most common organisms and identified in 25 (80.6%) of 31 cases with surgical site infections. The surgical site infection rate in the vancomycin powder group (0/105, 0.0%) was significantly lower than that in the conventional group (31/475, 6.5%; crude odds ratio [OR], 0.067; 95% confidence interval [CI], 0.006–0.762; adjusted OR, 0.068; 95% CI, 0.006–0.731; p=0.026). No adverse events associated with intrawound vancomycin powder were observed during the follow-up.
Conclusion
: Intrawound vancomycin powder effectively prevented surgical site infections following cranioplasty without local or systemic adverse events. Our results suggest that intrawound vancomycin powder is an effective and safe strategy for patients undergoing cranioplasty.
Keyword
Figure
Reference
-
References
1. Abode-Iyamah KO, Chiang HY, Winslow N, Park B, Zanaty M, Dlouhy BJ, et al. Risk factors for surgical site infections and assessment of vancomycin powder as a preventive measure in patients undergoing first-time cranioplasty. J Neurosurg. 128:1241–1249. 2018.2. Bader ER, Kobets AJ, Ammar A, Goodrich JT. Factors predicting complications following cranioplasty. J Craniomaxillofac Surg. 50:134–139. 2022.3. Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 152:784–791. 2017.4. Coulter IC, Pesic-Smith JD, Cato-Addison WB, Khan SA, Thompson D, Jenkins AJ, et al. Routine but risky: a multi-centre analysis of the outcomes of cranioplasty in the Northeast of England. Acta Neurochir (Wien). 156:1361–1368. 2014.5. Devin CJ, Chotai S, McGirt MJ, Vaccaro AR, Youssef JA, Orndorff DG, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: a multicenter analysis. Spine (Phila Pa 1976). 43:65–71. 2018.6. Dial BL, Lampley AJ, Green CL, Hallows R. Intrawound vancomycin powder in primary total hip arthroplasty increases rate of sterile wound complications. Hip Pelvis. 30:37–44. 2018.7. Donovan TJ, Sino S, Paraforos A, Leick J, Friedrich I. Topical vancomycin reduces the incidence of deep sternal wound complications after sternotomy. Ann Thorac Surg. 114:511–518. 2022.8. Ghobrial GM, Cadotte DW, Williams K Jr, Fehlings MG, Harrop JS. Complications from the use of intrawound vancomycin in lumbar spinal surgery: a systematic review. Neurosurg Focus. 39:E11. 2015.9. Goedemans T, Verbaan D, van der Veer O, Bot M, Post R, Hoogmoed J, et al. Complications in cranioplasty after decompressive craniectomy: timing of the intervention. J Neurol. 267:1312–1320. 2020.10. Haimoto S, Schär RT, Nishimura Y, Hara M, Wakabayashi T, Ginsberg HJ. Reduction in surgical site infection with suprafascial intrawound application of vancomycin powder in instrumented posterior spinal fusion: a retrospective case-control study. J Neurosurg Spine. 29:193–198. 2018.11. Hanada M, Nishikino S, Hotta K, Furuhashi H, Hoshino H, Matsuyama Y. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Arthrosc. 27:2322–2327. 2019.12. Honeybul S, Ho KM. Cranioplasty: morbidity and failure. Br J Neurosurg. 30:523–528. 2016.13. Im SH, Jang DK, Han YM, Kim JT, Chung DS, Park YS. Long-term incidence and predicting factors of cranioplasty infection after decompressive craniectomy. J Korean Neurosurg Soc. 52:396–403. 2012.14. Jeong TS, Yee GT. Prospective multicenter surveillance study of surgical site infection after intracranial procedures in Korea : a preliminary study. J Korean Neurosurg Soc. 61:645–652. 2018.15. Kim JS, Park IS, Kim SK, Park H, Kang DH, Lee CH, et al. Analysis of the risk factors affecting the surgical site infection after cranioplasty following decompressive craniectomy. Korean J Neurotrauma. 11:100–105. 2015.16. Kim YM, Park T, Lee SP, Baek JW, Ryou KS, Kim SH. Optimal timing and complications of cranioplasty: a single-center retrospective review of 109 cases. J Neurointensive Care. 3:48–57. 2020.17. Major Extremity Trauma Research Consortium (METRC), O'Toole RV, Joshi M, Carlini AR, Murray CK, Allen LE, et al. Effect of intrawound vancomycin powder in operatively treated high-risk tibia fractures: a randomized clinical trial. JAMA Surg. 156:e207259. 2021.18. Molinari RW, Khera OA, Molinari WJ 3rd. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J 21 Suppl. 4:S476–S482. 2012.19. Morton RP, Abecassis IJ, Hanson JF, Barber J, Nerva JD, Emerson SN, et al. Predictors of infection after 754 cranioplasty operations and the value of intraoperative cultures for cryopreserved bone flaps. J Neurosurg. 125:766–770. 2016.20. National Healthcare Safety Network. Center for Disease Control and Prevention : Surgical Site Infection (SSI) Event. Available at : https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.21. Patel NN, Guild GN 3rd, Kumar AR. Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today. 4:479–483. 2018.22. Ravikumar V, Ho AL, Pendhakar AV, Sussman ES, Kwong-Hon Chow K, Li G. The use of vancomycin powder for surgical prophylaxis following craniotomy. Neurosurgery. 80:754–758. 2017.23. Riordan MA, Simpson VM, Hall WA. Analysis of factors contributing to infections after cranioplasty: a single-institution retrospective chart review. World Neurosurg. 87:207–213. 2016.24. Saleh A, Thabet A, Belkhair S. Topical vancomycin for prevention of surgical site infection after craniotomy: meta-analysis and systematic literature review. World Neurosurg. 158:e605–e611. 2022.25. Shah SJ, Jadhav UE. Efficacy of topical vancomycin application in cardiac surgery to reduce deep sternal wound infection: a randomised control trial at tertiary cardiac care hospital. Int Surg J. 9:601–605. 2022.26. Shibahashi K, Hoda H, Takasu Y, Hanakawa K, Ide T, Hamabe Y. Cranioplasty outcomes and analysis of the factors influencing surgical site infection: a retrospective review of more than 10 years of institutional experience. World Neurosurg. 101:20–25. 2017.27. Sundseth J, Sundseth A, Berg-Johnsen J, Sorteberg W, Lindegaard KF. Cranioplasty with autologous cryopreserved bone after decompressive craniectomy: complications and risk factors for developing surgical site infection. Acta Neurochir (Wien). 156:805–811. discussion 811. 2014.28. Ushirozako H, Hasegawa T, Yamato Y, Yoshida G, Yasuda T, Banno T, et al. Impact of intrawound vancomycin powder on prevention of surgical site infection after posterior spinal surgery. J Neurosurg Spine. 34:656–664. 2021.29. Zanaty M, Chalouhi N, Starke RM, Chitale R, Hann S, Bovenzi CD, et al. Predictors of infections following cranioplasty: a retrospective review of a large single center study. ScientificWorldJournal. 2014:356042. 2014.30. Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M, et al. Complications following cranioplasty: incidence and predictors in 348 cases. J Neurosurg. 123:182–188. 2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prophylactic Intrawound Application of Vancomycin Powder in Instrumented Spinal Fusion Surgery
- Do Dose-Dependent Microbial Changes Occur during Spine Surgery as a Result of Applying Intrawound Vancomycin Powder?: A Systematic Literature Review
- Effect of Using Local Intrawound Vancomycin Powder in Addition to Intravenous Antibiotics in Posterior Lumbar Surgery: Midterm Result in a Single-Center Study
- Delayed and recurrent surgical site infection from resorbed bone fragment after autologous cranioplasty: a case report
- Does the Application of Topical Intrawound Vancomycin Powder Affect Deep Surgical Site Infection and the Responsible Organisms after Spinal Surgery?: A Retrospective Case Series with a Historical Control Group