Clin Exp Otorhinolaryngol.
2023 Aug;16(3):225-235. 10.21053/ceo.2023.00227.
Effects of Particulate Matter Exposure on the Eustachian Tube and Middle Ear Mucosa of Rats
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University College of Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 2The Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 3Department of Environmental Engineering, Pukyong National University, Busan, Korea
- 4Department of Pathology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University College of Medicine, Pusan National University Hospital, Busan, Korea
- 6Department of Otorhinolaryngology, Inje University College of Medicine, Haeundae Paik Hospital, Busan, Korea
- KMID: 2545261
- DOI: http://doi.org/10.21053/ceo.2023.00227
Abstract
Objectives
. Particulate matter (PM) is a risk factor for various diseases. Recent studies have established an association between otitis media (OM) and PM exposure. To confirm this relationship, we developed a novel exposure model designed to control the concentration of PM, and we observed the effects of PM exposure on the Eustachian tube (ET) and middle ear mucosa of rats.
Methods
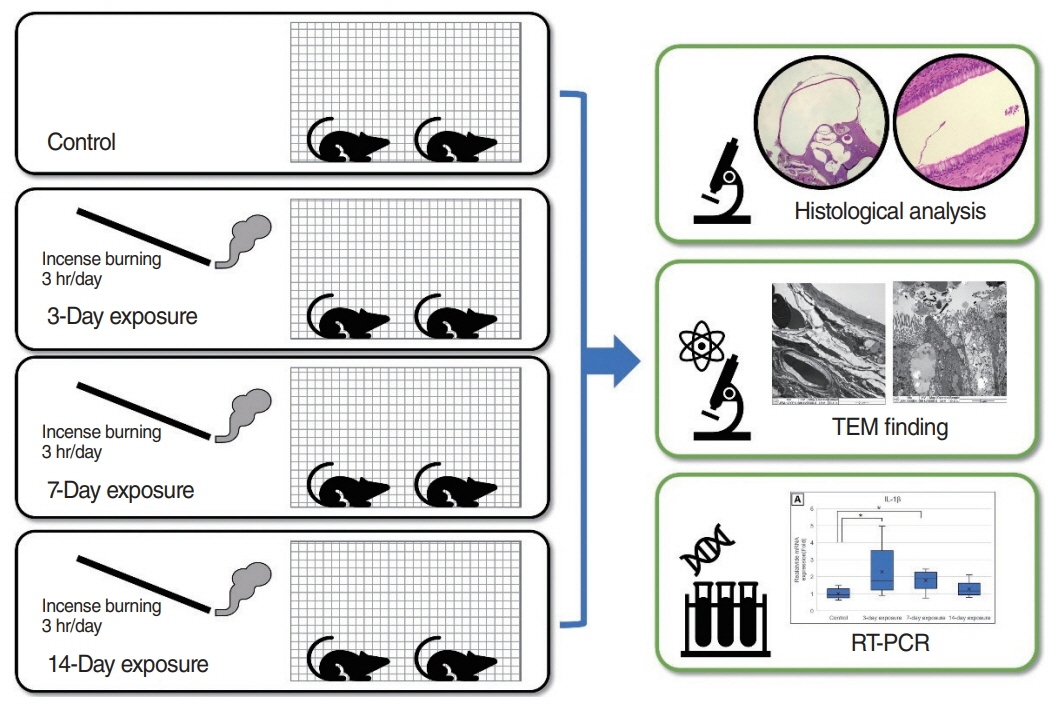
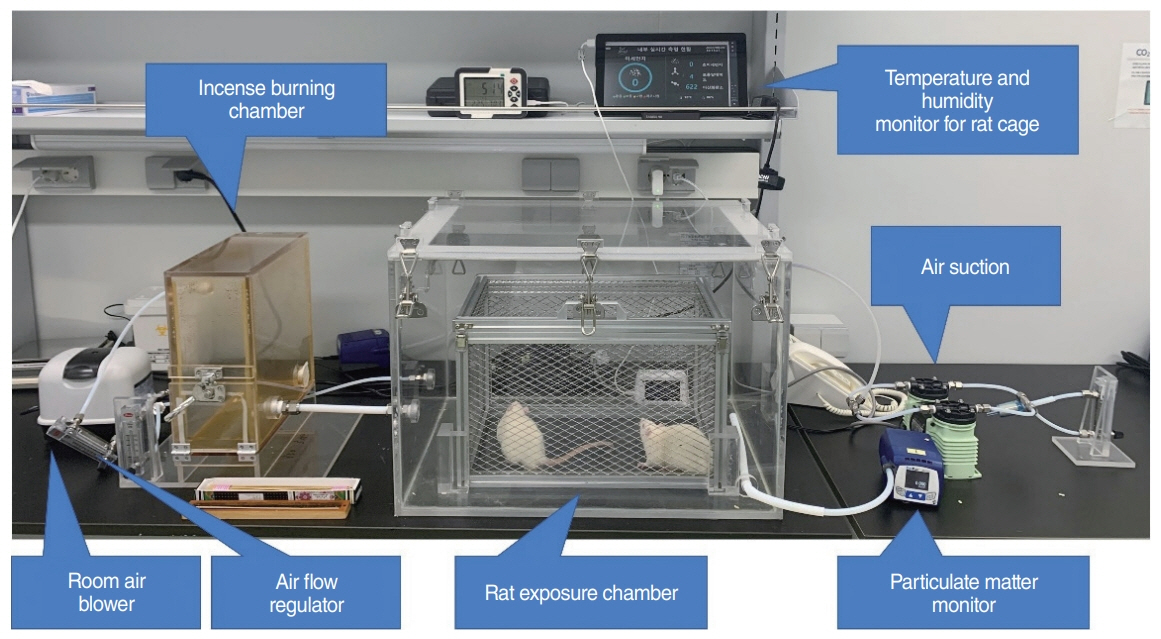
. Forty healthy, 10-week-old, male Sprague-Dawley rats were divided into 3-day, 7-day, 14-day exposure, and control groups (each, n=10). The rats were exposed to incense smoke as the PM source for 3 hours per day. After exposure, bilateral ETs and mastoid bullae were harvested, and histopathological findings were compared using microscopy and transmission electron microscopy (TEM). The expression levels of interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and vascular endothelial growth factor (VEGF) in the middle ear mucosa of each group were compared using real-time reverse transcription polymerase chain reaction (RT-PCR).
Results
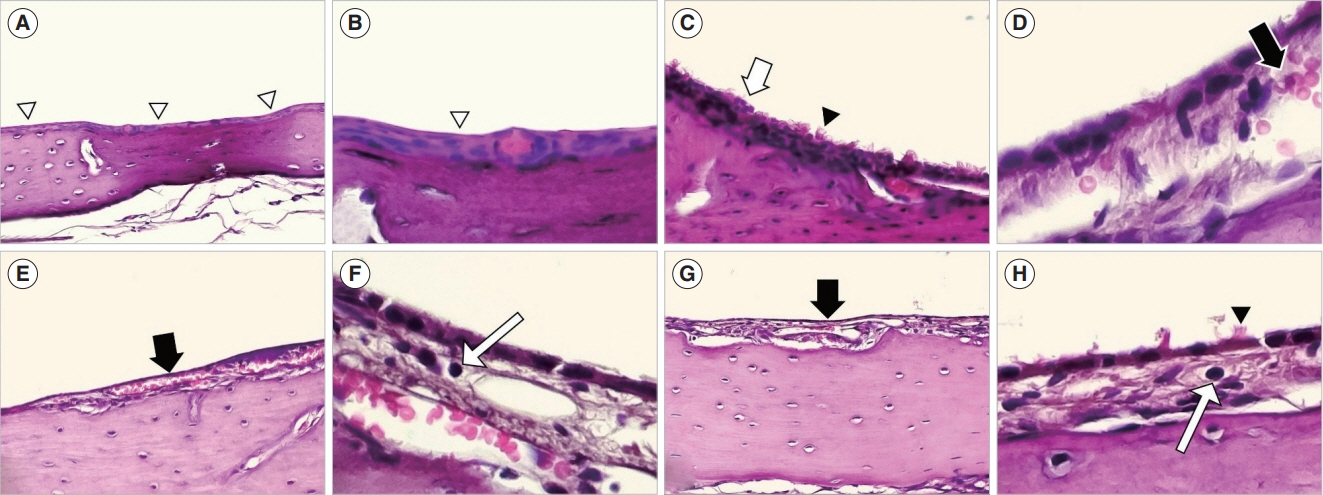
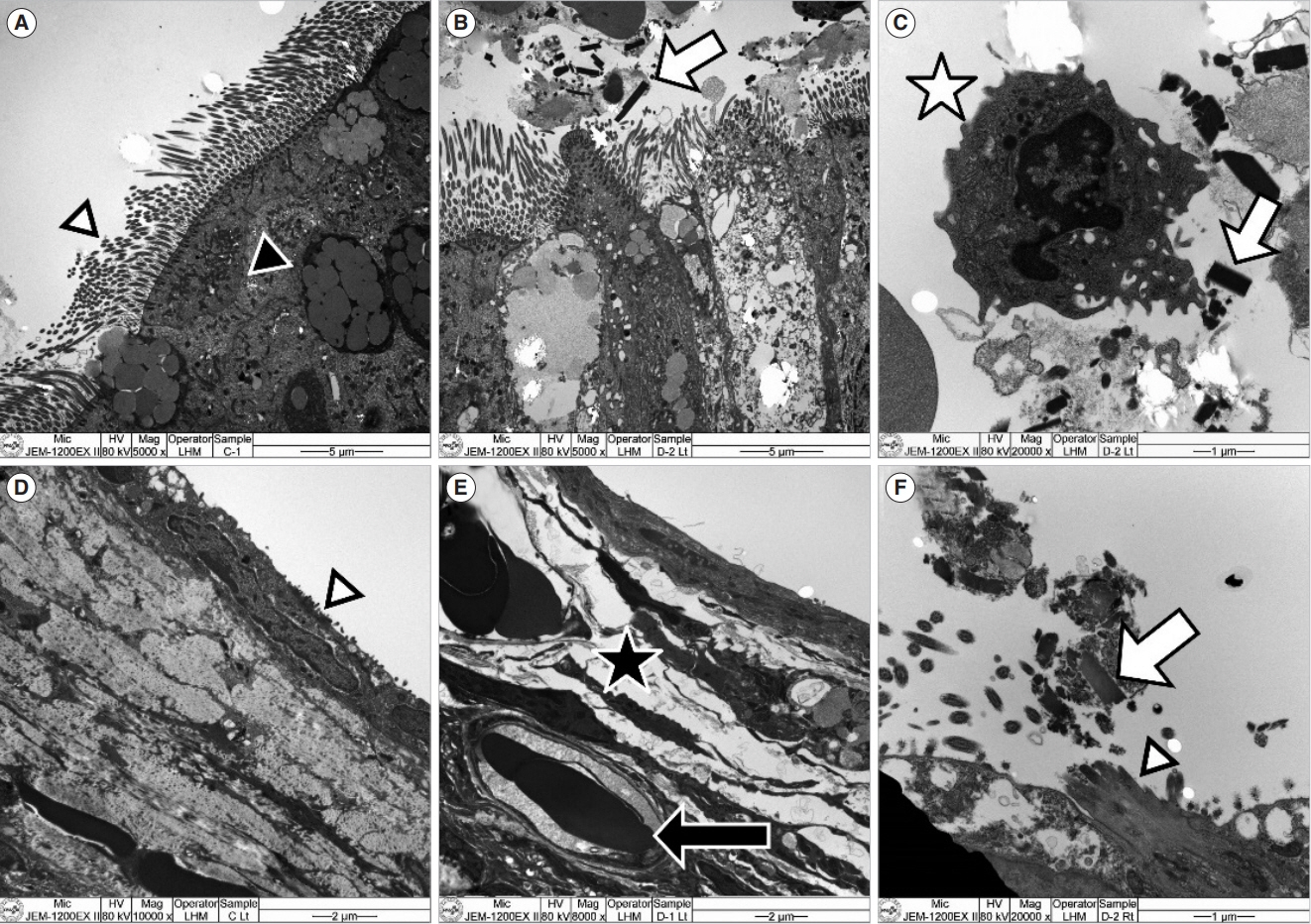
. In the ET mucosa of the exposure group, the goblet cell count significantly increased after PM exposure (P=0.032). In the middle ear mucosa, subepithelial space thickening, increased angio-capillary tissue, and inflammatory cell infiltration were observed. Moreover, the thickness of the middle ear mucosa in the exposure groups increased compared to the control group (P<0.01). The TEM findings showed PM particles on the surface of the ET and middle ear mucosa, and RT-PCR revealed that messenger RNA (mRNA) expression of IL-1β significantly increased in the 3-day and 7-day exposure groups compared to the control group (P=0.035). VEGF expression significantly increased in the 7-day exposure group compared to the control and 3-day exposure groups (P<0.01).
Conclusion
. The ET and middle ear mucosa of rats showed histopathologic changes after acute exposure to PM that directly reached the ET and middle ear mucosa. Therefore, acute exposure to PM may play a role in the development of OM.
Keyword
Figure
Cited by 1 articles
-
Reprogramming Macrophage Phenotypes With Photobiomodulation for Improved Inflammation Control in ENT Organ Tissues
Ken Woo, Yeon Soo Kim, Celine Abueva, Seung Hoon Woo
Clin Exp Otorhinolaryngol. 2025;18(1):1-13. doi: 10.21053/ceo.2024.00286.
Reference
-
1. World Health Organization. Ambient (outdoor) air pollution [Internet]. World Health Organization;2022. [cited 2023 May 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.2. Thompson JE. Airborne particulate matter: human exposure and health effects. J Occup Environ Med. 2018; May. 60(5):392–423.3. Park M, Han J, Jang MJ, Suh MW, Lee JH, Oh SH, et al. Air pollution influences the incidence of otitis media in children: a national population-based study. PLoS One. 2018; Jun. 13(6):e0199296.4. Naclerio R, Ansotegui IJ, Bousquet J, Canonica GW, D’Amato G, Rosario N, et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: impact of air pollution on patients with AR: current knowledge and future strategies. World Allergy Organ J. 2020; Apr. 13(3):100106.5. Park M, Han J, Park J, Jang MJ, Park MK. Particular matter influences the incidence of acute otitis media in children. Sci Rep. 2021; Oct. 11(1):19730.6. Zhang Z, Rowan NR, Pinto JM, London NR, Lane AP, Biswal S, et al. Exposure to particulate matter air pollution and anosmia. JAMA Netw Open. 2021; May. 4(5):e2111606.7. Coco A, Vernacchio L, Horst M, Anderson A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010; Feb. 125(2):214–20.8. Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012; 7(4):e36226.9. Rovers MM. The burden of otitis media. Vaccine. 2008; Dec. 26 Suppl 7:G2–4.10. Bowatte G, Tham R, Perret JL, Bloom MS, Dong G, Waidyatillake N, et al. Air pollution and otitis media in children: a systematic review of literature. Int J Environ Res Public Health. 2018; Feb. 15(2):257.11. Lee SY, Jang MJ, Oh SH, Lee JH, Suh MW, Park MK. Associations between particulate matter and otitis media in children: a meta-analysis. Int J Environ Res Public Health. 2020; Jun. 17(12):4604.12. Park MK, Chae SW, Kim HB, Cho JG, Song JJ. Middle ear inflammation of rat induced by urban particles. Int J Pediatr Otorhinolaryngol. 2014; Dec. 78(12):2193–7.13. Yadav MK, Go YY, Chae SW, Park MK, Song JJ. Asian sand dust particles increased pneumococcal biofilm formation in vitro and colonization in human middle ear epithelial cells and rat middle ear mucosa. Front Genet. 2020; Apr. 11:323.14. Yoon CS, Jun NI, Kim DY, Kim MK, Kim JL. A study on areal & dimensional characteristics of 21C apartment typical unit plans in Seoul and its metropolitan vicinity. J Korean Hous Assoc. 2008; Dec. 19(6):21–32.15. Hussain T, Al-Attas OS, Al-Daghri NM, Mohammed AA, De Rosas E, Ibrahim S, et al. Induction of CYP1A1, CYP1A2, CYP1B1, increased oxidative stress and inflammation in the lung and liver tissues of rats exposed to incense smoke. Mol Cell Biochem. 2014; Jun. 391(1-2):127–36.16. Hussain T, Alamery S, Dikshit G, Mohammed AA, Naushad SM, Alrokayan S. Incense smoke exposure augments systemic oxidative stress, inflammation and endothelial dysfunction in male albino rats. Toxicol Mech Methods. 2019; Mar. 29(3):211–8.17. Yamamoto N, Kan-O K, Tatsuta M, Ishii Y, Ogawa T, Shinozaki S, et al. Incense smoke-induced oxidative stress disrupts tight junctions and bronchial epithelial barrier integrity and induces airway hyperresponsiveness in mouse lungs. Sci Rep. 2021; Mar. 11(1):7222.18. Choi SW, Choi S, Kang EJ, Lee HM, Oh SJ, Lee IW, et al. Effects of cigarette smoke on Haemophilus influenzae-induced otitis media in a rat model. Sci Rep. 2021; Oct. 11(1):19729.19. Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, et al. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res. 2005; Sep. 99(1):40–7.20. Chauhan AJ, Johnston SL. Air pollution and infection in respiratory illness. Br Med Bull. 2003; Dec. 68(1):95–112.21. Fang GC, Chang CN, Chu CC, Wu YS, Pi-Cheng Fu P, Chang SC, et al. Fine (PM2.5), coarse (PM2.5-10), and metallic elements of suspended particulates for incense burning at Tzu Yun Yen temple in central Taiwan. Chemosphere. 2003; Jun. 51(9):983–91.22. Mannix RC, Nguyen KP, Tan EW, Ho EE, Phalen RF. Physical characterization of incense aerosols. Sci Total Environ. 1996; Dec. 193(2):149–58.23. Lin TC, Krishnaswamy G, Chi DS. Incense smoke: clinical, structural and molecular effects on airway disease. Clin Mol Allergy. 2008; Apr. 6:3.24. Yang CY, Chiu JF, Cheng MF, Lin MC. Effects of indoor environmental factors on respiratory health of children in a subtropical climate. Environ Res. 1997; Oct. 75(1):49–55.25. Ho CK, Tseng WR, Yang CY. Adverse respiratory and irritant health effects in temple workers in Taiwan. J Toxicol Environ Health A. 2005; Sep. 68(17-18):1465–70.26. Friborg JT, Yuan JM, Wang R, Koh WP, Lee HP, Yu MC. Incense use and respiratory tract carcinomas: a prospective cohort study. Cancer. 2008; Oct. 113(7):1676–84.27. Juhn SK, Jung MK, Hoffman MD, Drew BR, Preciado DA, Sausen NJ, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008; Sep. 1(3):117–38.28. Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Perspect. 2018; Jan. 126(1):017007.29. Jung HH, Kim MW, Lee JH, Kim YT, Kim NH, Chang BA, et al. Expression of vascular endothelial growth factor in otitis media. Acta Otolaryngol. 1999; 119(7):801–8.30. Melhus A, Ryan AF. Expression of cytokine genes during pneumococcal and nontypeable Haemophilus influenzae acute otitis media in the rat. Infect Immun. 2000; Jul. 68(7):4024–31.31. Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999; Jan. 340(4):260–4.32. Wang S, Chen Y, Hong W, Li B, Zhou Y, Ran P. Chronic exposure to biomass ambient particulate matter triggers alveolar macrophage polarization and activation in the rat lung. J Cell Mol Med. 2022; Feb. 26(4):1156–68.33. Cho SY, So WY, Roh HT. Impact of particulate matter exposure duration and intensity on circulating pro-inflammatory cytokines. Iran J Public Health. 2022; Feb. 51(2):460–2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Eustachian Tube Mature Teratoma
- A Case of Subcutaneous Emphysema and Pneumomediastinum After Balloon Eustachian Tuboplasty
- Role of Superoxide Dismutase in Eustachian Tube of Rat with Acute Otitis Media
- Immunohistochemical Localization of Alpha 1-antitrypsin in the Rat Eustachian Tube and Tympanic Membrane
- Imaging Finding of Malignant Melanoma of Eustachian Tube with Extension to Middle Ear Cavity: Case Report