Brain Tumor Res Treat.
2023 Jul;11(3):159-165. 10.14791/btrt.2023.0001.
Medical Applications of 3D Printing and Standardization Issues
- Affiliations
-
- 1Department of Pediatric Neurosurgery, Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2545111
- DOI: http://doi.org/10.14791/btrt.2023.0001
Abstract
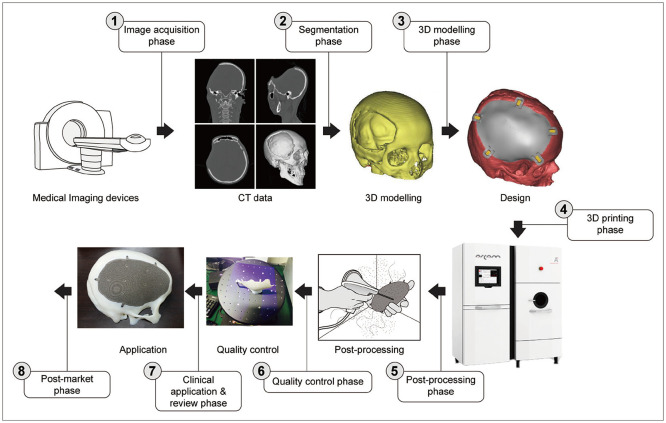
- The three-dimensional (3D) printing itself is not a novel technology, it is more than 30 years old. Stereolithographic (SLA) technology has been used as the first and popular technology for medical application of 3D printing. Since 1991 Radiology and Plastic Surgery have published articles about SLA for rapid prototyping anatomical 3D models. Medical applications of 3D printing have been popularizing and stabilizing so far. Implantable medical devices such as metal or absorbable implants, surgical guide systems, prosthesis and orthosis, and 3D anatomical models for normal or diseased anatomy have been developing and expanding its markets so far. There are many obstacles, such as insurance, authorization as a medical device, and lack of standards technology for further expansion of medical applications. Many technical specifications and guidelines for authorization as medical device have been published by regulatory bodies from many countries. Even though international standards for 3D printing have been developing more and more, there have been few standards for medical application of 3D printing. In this harsh environment academia, company, research institute, regulatory bodies, and government have been doing good job for the development of 3D printing industry.
Keyword
Figure
Reference
-
1. Ashley S. Rapid prototyping systems. Mech Eng. 1991; 113:34–43.2. Barker TM, Earwaker WJ, Frost N, Wakeley G. Integration of 3-D medical imaging and rapid prototyping to create stereolithographic models. Australas Phys Eng Sci Med. 1993; 16:79–85. PMID: 8357307.3. Mankovich N, Yue A, Ammirati M, Kioumehr F, Turner S. Solid models for CT/MR image display: accuracy and utility in surgical planning. In : Proceedings of Medical Imaging ‘91; 1991 Feb 24-28; San Jose, CA, USA. SPIE;1991.4. Radiological Society of North America. 3D printing special interest group [Internet]. Oak Brook, IL: Radiological Society of North America;Accessed Dec 27, 2022. at: https://www.rsna.org/membership/involvement-opportunities/3d-printing-special-interest-group.5. D’Urso PS, Atkinson RL, Lanigan MW, Earwaker WJ, Bruce IJ, Holmes A, et al. Stereolithographic (SL) biomodelling in craniofacial surgery. Br J Plast Surg. 1998; 51:522–530. PMID: 9924405.6. Cho HR, Roh TS, Shim KW, Kim YO, Lew DH, Yun IS. Skull reconstruction with custom made three-dimensional titanium implant. Arch Craniofac Surg. 2015; 16:11–16. PMID: 28913212.7. D’Urso PS, Earwaker WJ, Barker TM, Redmond MJ, Thompson RG, Effeney DJ, et al. Custom cranioplasty using stereolithography and acrylic. Br J Plast Surg. 2000; 53:200–204. PMID: 10738323.8. Winder J, Cooke RS, Gray J, Fannin T, Fegan T. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol. 1999; 23:26–28. PMID: 10202700.9. Park EK, Lim JY, Yun IS, Kim JS, Woo SH, Kim DS, et al. Cranioplasty enhanced by three-dimensional printing: custom-made three-dimensional-printed titanium implants for skull defects. J Craniofac Surg. 2016; 27:943–949. PMID: 27192643.10. Park SE, Park EK, Shim KW, Kim DS. Modified cranioplasty technique using 3-dimensional printed implants in preventing temporalis muscle hollowing. World Neurosurg. 2019; 126:e1160–e1168. PMID: 30880206.11. ISO. ISO/ASTM 52900:2021. Additive manufacturing — General principles — Fundamentals and Vocabulary [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/74514.html.12. Durand JL, Renier D, Marchac D. [The history of cranioplasty]. Ann Chir Plast Esthet. 1997; 42:75–83. French. PMID: 9768140.13. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997; 47:238–241. PMID: 9068693.14. Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999; 9:271–278. PMID: 10525845.15. ISO. ISO/IEC 3532-1:2023. Information technology — Medical image-based modelling for 3D printing — Part 1: general requirements [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/79624.html.16. Han HH, Shim JH, Lee H, Kim BY, Lee JS, Jung JW, et al. Reconstruction of complex maxillary defects using patient-specific 3D-printed biodegradable scaffolds. Plast Reconstr Surg Glob Open. 2018; 6:e1975. PMID: 30881789.17. Jung BK, Kim JY, Kim YS, Roh TS, Seo A, Park KH, et al. Ideal scaffold design for total ear reconstruction using a three-dimensional printing technique. J Biomed Mater Res B Appl Biomater. 2019; 107:1295–1303. PMID: 30261122.18. Lee S, Choi D, Shim JH, Nam W. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci Rep. 2020; 10:4979. PMID: 32188900.19. Lee JS, Kim BS, Seo D, Park JH, Cho DW. Three-dimensional cell printing of large-volume tissues: application to ear regeneration. Tissue Eng Part C Methods. 2017; 23:136–145. PMID: 28093047.20. Lee JM, Sultan MT, Kim SH, Kumar V, Yeon YK, Lee OJ, et al. Artificial auricular cartilage using silk fibroin and polyvinyl alcohol hydrogel. Int J Mol Sci. 2017; 18:1707. PMID: 28777314.21. Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop Relat Res. 2013; 471:750–761. PMID: 22948530.22. Cho HS, Park YK, Gupta S, Yoon C, Han I, Kim HS, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017; 6:137–143. PMID: 28258117.23. Wong KC, Kumta SM, Sze KY, Wong CM. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput Aided Surg. 2012; 17:284–293. PMID: 23030839.24. Park JW, Kang HG, Lim KM, Park DW, Kim JH, Kim HS. Bone tumor resection guide using three-dimensional printing for limb salvage surgery. J Surg Oncol. 2018; 118:898–905. PMID: 30261096.25. Dong C, Beglinger I, Krieg AH. Personalized 3D-printed guide in malignant bone tumor resection and following reconstruction - 17 cases in pelvic and extremities. Surg Oncol. 2022; 42:101733. PMID: 35397377.26. ISO. ISO 13485:2016. Medical devices — Quality management systems — Requirements for regulatory purposes [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/59752.html.27. Ministry of Food and Drug Safety. Good manufacturing practices (GMP) for custom made medical device manufactured by 3D printing [Internet]. Cheongju: Ministry of Food and Drug Safety;Accessed Dec 27, 2022. at: https://www.mfds.go.kr/brd/m_1060/view.do?seq=14360.28. FDA. Technical considerations for additive manufactured medical devices [Internet]. Silver Spring, MD: FDA;Accessed Dec 27, 2022. at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-additive-manufactured-medical-devices.29. ISO. ISO/IEC DIS 3532-2. Information technology — Medical image-based modelling for 3D printing — Part 2: segmentation [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/79625.html.30. ISO. ISO/IEC AWI 8803. Information technology — 3D printing and scanning — Accuracy and precision evaluation process for modeling from 3D scanned data [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/84598.html.31. ISO. ISO/IEC AWI 16466. Information technology — 3D printing and scanning — Assessment methods of 3D scanned data for 3D printing model [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/84599.html.32. ISO. ISO/IEC AWI 8801. Information technology — 3D printing and scanning— 3D scanned and labeled data standard operating procedure (SOP) for evaluation of modelling from 3D scanned data [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/84597.html.33. ISO. ISO/AWI 5092. Additive manufacturing for medical — General principles — Roadmap to safe and effective additively manufactured implants [Internet]. Geneva: ISO;Accessed Dec 27, 2022. at: https://www.iso.org/standard/80762.html.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Three-Dimensional Printing in the Fracture Management
- A Review of Current Clinical Applications of Three-Dimensional Printing in Spine Surgery
- Clinical Applications of Three-Dimensional Printing in Cardiovascular Disease
- Three-Dimensional Printing Technology in Orthopedic Surgery
- Surface Functionalization of Three-Dimensional Printed Scaffold for Biomedical Application