J Korean Med Sci.
2023 Aug;38(31):e242. 10.3346/jkms.2023.38.e242.
Properties of Pleural Mesothelial Cells in Idiopathic Pulmonary Fibrosis and Cryptogenic Organizing Pneumonia
- Affiliations
-
- 1Department of Internal Medicine, Myongji Hospital, Hanyang University, Goyang, Korea
- 2German Hospital, Tirana, Albania
- 3Department of Pathology, Konyang University Hospital, Daejeon, Korea
- KMID: 2544961
- DOI: http://doi.org/10.3346/jkms.2023.38.e242
Abstract
- Background
Profibrotic properties of pleural mesothelial cells may play an important role in the fibrosis activity in idiopathic pulmonary fibrosis (IPF). The purpose of this study was to compare the expression of pleural mesothelial cell markers in IPF and cryptogenic organizing pneumonia (COP), with an assumption that increased expression implies increase in fibrosis.
Methods
Twenty IPF lung samples were stained by immunohistochemistry for the pleural mesothelial cell markers: leucine rich repeat neuronal 4 (LRRN4), uroplakin 3B, CCchemokine ligand 18, and laminin-5. Nine COP lung samples were used as controls. A semiquantitative analysis was performed to compare markers expression in IPF and COP.
Results
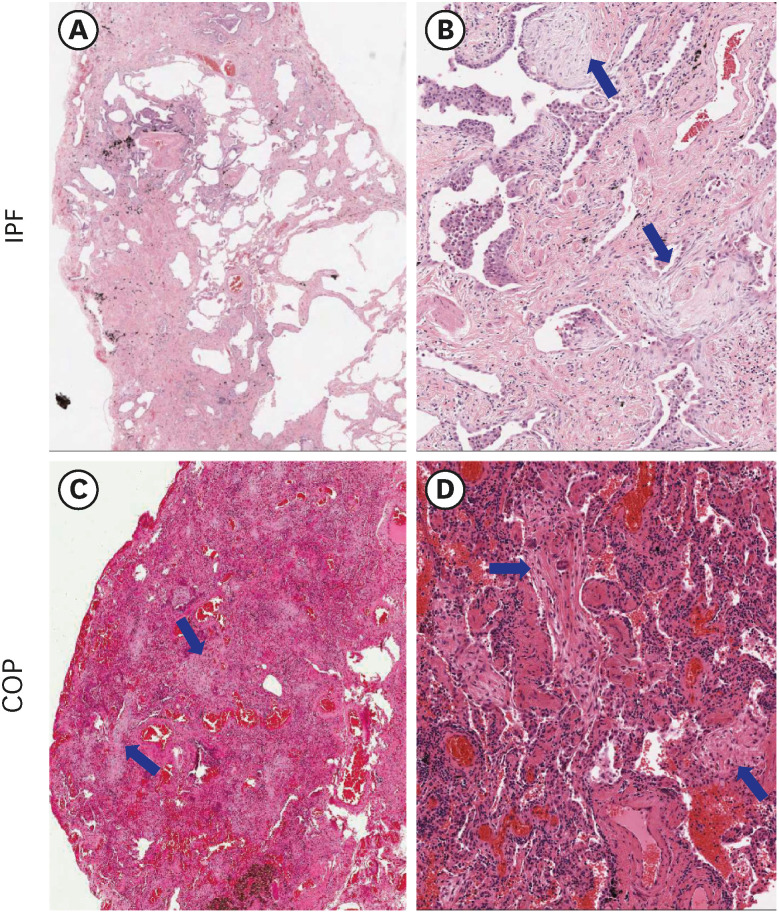
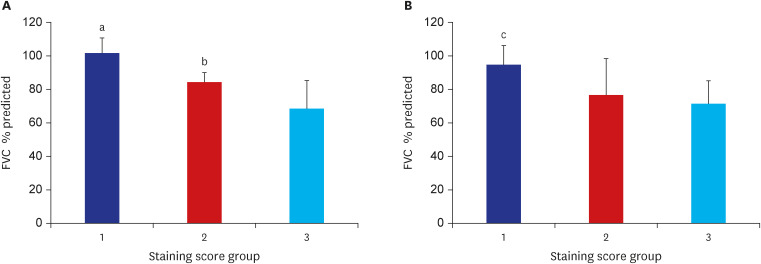
LRRN4 expression was found in epithelial lining cells along the honeycombing and fibroblastic foci in IPF, but not in the fibrotic interstitial lesion and airspace filling fibrous tufts in COP. We found a significant decrease in baseline forced vital capacity when LRRN4 expression was increased in honeycombing epithelial cells and fibroblastic foci.
Conclusion
LRRN4 expression patterns in IPF are distinct from those in COP. Our findings suggest that mesothelial cell profibrotic property may be an important player in IPF pathogenesis and may be a clue in the irreversibility of fibrosis in IPF.
Figure
Reference
-
1. Hadjicharalambous MR, Lindsay MA. Idiopathic pulmonary fibrosis: pathogenesis and the emerging role of long non-coding RNAs. Int J Mol Sci. 2020; 21(2):21.2. Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018; 19(1):32. PMID: 29471816.3. Mubarak KK, Montes-Worboys A, Regev D, Nasreen N, Mohammed KA, Faruqi I, et al. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur Respir J. 2012; 39(1):133–140. PMID: 21737551.4. Liu F, Yu F, Lu YZ, Cheng PP, Liang LM, Wang M, et al. Crosstalk between pleural mesothelial cell and lung fibroblast contributes to pulmonary fibrosis. Biochim Biophys Acta Mol Cell Res. 2020; 1867(11):118806. PMID: 32739525.5. Tanabe N, McDonough JE, Vasilescu DM, Ikezoe K, Verleden SE, Xu F, et al. Pathology of idiopathic pulmonary fibrosis assessed by a combination of microcomputed tomography, histology, and immunohistochemistry. Am J Pathol. 2020; 190(12):2427–2435. PMID: 32919981.6. Fabro AT, Minatel IO, Rangel MP, Halbwedl I, Parra ER, Capelozzi VL, et al. Usual interstitial pneumonia and smoking-related interstitial fibrosis display epithelial to mesenchymal transition in fibroblastic foci. Respir Med. 2014; 108(9):1377–1386. PMID: 25066934.7. Chandra D, Maini R, Hershberger DM. Cryptogenic Organizing Pneumonia. Treasure Island, FL, USA: StatPearls;2022.8. Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med. 2012; 33(5):462–475. PMID: 23001801.9. Batra H, Antony VB. Pleural mesothelial cells in pleural and lung diseases. J Thorac Dis. 2015; 7(6):964–980. PMID: 26150910.10. Kanamori-Katayama M, Kaiho A, Ishizu Y, Okamura-Oho Y, Hino O, Abe M, et al. LRRN4 and UPK3B are markers of primary mesothelial cells. PLoS One. 2011; 6(10):e25391. PMID: 21984916.11. Schraufstatter IU, Zhao M, Khaldoyanidi SK, Discipio RG. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology. 2012; 135(4):287–298. PMID: 22117697.12. Lappi-Blanco E, Kaarteenaho-Wiik R, Salo S, Sormunen R, Määttä M, Autio-Harmainen H, et al. Laminin-5 gamma2 chain in cryptogenic organizing pneumonia and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004; 169(1):27–33. PMID: 14500258.13. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17(5):1474–1481. PMID: 10334533.14. Miller-Hodges E, Hohenstein P. WT1 in disease: shifting the epithelial-mesenchymal balance. J Pathol. 2012; 226(2):229–240. PMID: 21959952.15. Batra H, Antony VB. The pleural mesothelium in development and disease. Front Physiol. 2014; 5:284. PMID: 25136318.16. Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009; 179(8):717–723. PMID: 19179488.17. Tiev KP, Hua-Huy T, Kettaneh A, Gain M, Duong-Quy S, Tolédano C, et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J. 2011; 38(6):1355–1360. PMID: 21778167.18. Hoffmann-Vold AM, Tennøe AH, Garen T, Midtvedt Ø, Abraityte A, Aaløkken TM, et al. High level of chemokine CCL18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest. 2016; 150(2):299–306. PMID: 26997242.19. Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, et al. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev. 2016; 96(4):1567–1591. PMID: 27630174.20. Zhang F, Ayaub EA, Wang B, Puchulu-Campanella E, Li YH, Hettiarachchi SU, et al. Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis. EMBO Mol Med. 2020; 12(8):e12034. PMID: 32597014.21. Song LJ, Zhou LL, Wang M, Liu F, Xiong L, Xiang F, et al. Lethal (2) giant larvae regulates pleural mesothelial cell polarity in pleural fibrosis. Biochim Biophys Acta BBAMol Cell Res. 2018; 1865(9):1201–1210.22. Chung JH, Goldin JG. Interpretation of HRCT scans in the diagnosis of ipf: improving communication between pulmonologists and radiologists. Lung. 2018; 196(5):561–567. PMID: 30097721.23. Palmucci S, Roccasalva F, Puglisi S, Torrisi SE, Vindigni V, Mauro LA, et al. Clinical and radiological features of idiopathic interstitial pneumonias (IIPs): a pictorial review. Insights Imaging. 2014; 5(3):347–364. PMID: 24844883.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Idiopathic Interstitial Pneumonias: Radiologic Findings
- Different Responses to Clarithromycin in Patients with Cryptogenic Organizing Pneumonia
- Radiologic Diagnosis of Interstitial Lung Diseases
- Radiologic Approach to the Idiopathic Interstitial Pneumonias
- 2008 National Survey of Idiopathic Interstitial Pneumonia in Korea