J Korean Med Sci.
2023 Jul;38(29):e232. 10.3346/jkms.2023.38.e232.
A Comparison of the Effects of Dexamethasone and Methylprednisolone, Used on Level-3 Intensive Care COVID-19 Patients, on Mortality: A Multi-Center Retrospective Study
- Affiliations
-
- 1Department of Anesthesiology and Reanimation/Intensive Care, Health Sciences University Haydarpasa Numune Training and Research Hospital, Istanbul, Turkey
- 2Department of Anesthesiology and Reanimation/Intensive Care, Health Sciences University Kartal Dr. Lutfi Kırdar Training and Research Hospital, Istanbul, Turkey
- 3Department of İnfectious Diseases and Clinical Microbiology, Health Sciences University Haydarpasa Numune Training and Research Hospital, Istanbul, Turkey
- 4Department of Anesthesiology and Reanimation/Intensive Care, Health Sciences University Bakırköy Dr. Sadi Konuk Training and Research Hospital, İstanbul, Turkey
- 5Department of Anesthesiology and Reanimation/Intensive Care, Health Sciences University Prof. Dr. Cemil Taşcıoğlu City Hospital, İstanbul, Turkey
- 6Department of Anesthesiology and Reanimation/Intensive Care, Health Sciences University Derince Training and Research Hospital, Kocaeli, Turkey
- 7Department of Anesthesiology and Reanimation/Intensive Care, Sancaktepe Şehit Prof Dr Ilhan Varank Training and Research Hospital, İstanbul, Turkey
- KMID: 2544946
- DOI: http://doi.org/10.3346/jkms.2023.38.e232
Abstract
- Background
Coronavirus disease 2019 (COVID-19) is often a mild disease, usually manifesting with respiratory complaints, and is sometimes mortal due to multiple organ failure. Hyperinflammation is a known COVID-19 component and is associated with organ dysfunction, disease severity and mortality. Controlling hyperinflammatory response is crucial in determining treatment direction. An important agent in providing this control is corticosteroids. This study aimed to determine whether dexamethasone and methylprednisolone, doses, administration time and duration in COVID-19 treatment are associated with improved treatment outcomes.
Methods
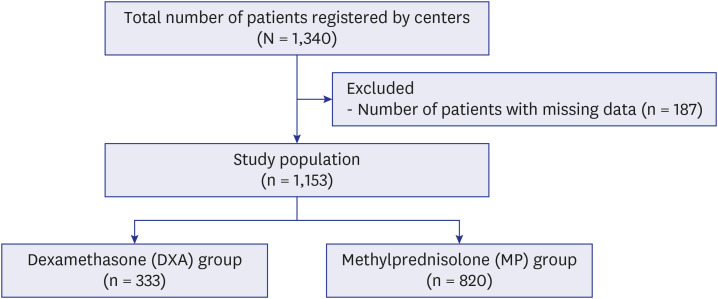
This retrospective multicenter study was conducted with participation of 6 healthcare centers which collected data by retrospectively examining files of 1,340 patients admitted to intensive care unit due to COVID-19 between March 2020 and September 2021, diagnosed with polymerase chain reaction (+) and/or clinically and radiologically.
Results
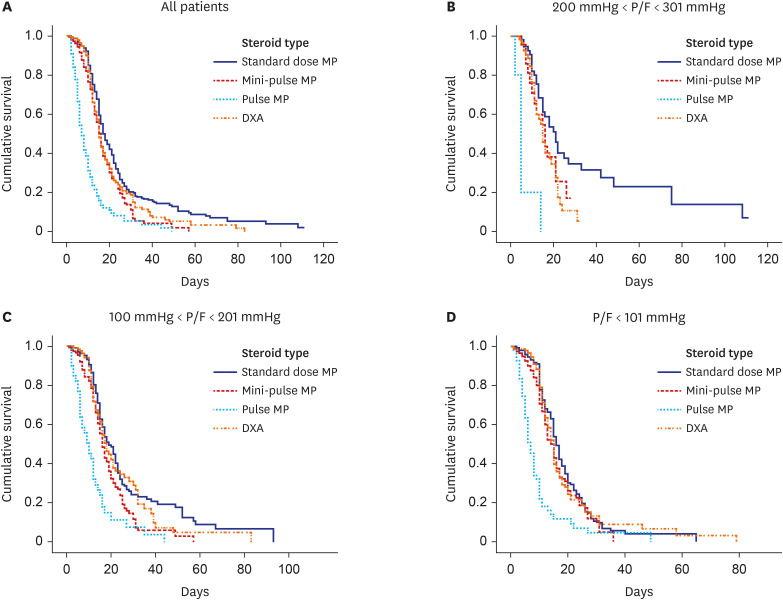
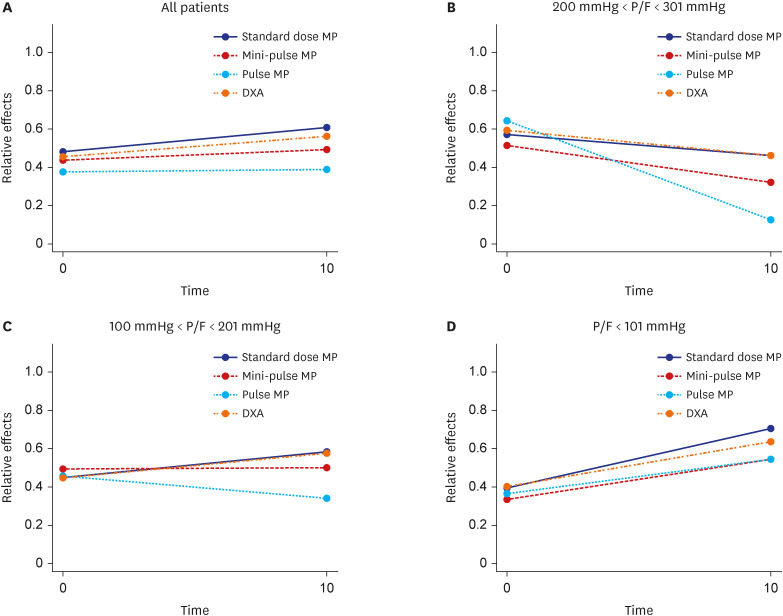
Mortality in the pulse methylprednisolone group was statistically significantly higher than that in the other 3 groups. Mortality was higher in older patients with comorbidities such as hypertension, diabetes mellitus, chronic kidney failure, coronary artery disease, and dementia. Pulse and mini-pulse steroid doses were less effective than standard methylprednisolone and dexamethasone doses, pulse steroid doses being associated with high mortality. Standard-dose methylprednisolone and dexamethasone led to similar effects, but standard dose methylprednisolone was more effective in severe patients who required mechanical ventilation (MV). Infection development was related to steroid treatment duration, not cumulative steroid dose.
Conclusion
Corticosteroids are shown to be beneficial in critical COVID-19, but the role of early corticosteroids in mild COVID-19 patients remains unclear. The anti-inflammatory effects of corticosteroids may have a positive effect by reducing mortality in severe COVID-19 patients. Although dexamethasone was first used for this purpose, methylprednisolone was found to be as effective at standard doses. Methylprednisolone administered at standard doses was associated with greater PaO 2 /FiO 2 ratios than dexamethasone, especially in the severe group requiring MV. High dose pulse steroid doses are closely associated with mortality and standard methylprednisolone dose is recommended.
Figure
Reference
-
1. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019; 11(8):762. PMID: 31430946.2. Dorward DA, Russell CD, Um IH, Elshani M, Armstrong SD, Penrice-Randal R, et al. Tissue-specific immunopathology in fatal COVID-19. Am J Respir Crit Care Med. 2021; 203(2):192–201. PMID: 33217246.3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229):1033–1034. PMID: 32192578.4. McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 disease. Am J Respir Crit Care Med. 2020; 202(6):812–821. PMID: 32584597.5. Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020; 56(6):2002808. PMID: 32943404.6. Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021; 897:173947. PMID: 33607104.7. Papamanoli A, Yoo J, Grewal P, Predun W, Hotelling J, Jacob R, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Invest. 2021; 51(2):e13458. PMID: 33219551.8. López Zúñiga MÁ, Moreno-Moral A, Ocaña-Granados A, Padilla-Moreno FA, Castillo-Fernández AM, Guillamón-Fernández D, et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021; 16(1):e0243964. PMID: 33507958.9. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020; 369:m1985. PMID: 32444460.10. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020; 324(13):1330–1341. PMID: 32876694.11. Kumar G, Patel D, Hererra M, Jefferies D, Sakhuja A, Meersman M, et al. Do high-dose corticosteroids improve outcomes in hospitalized COVID-19 patients? J Med Virol. 2022; 94(1):372–379. PMID: 34559436.12. Swaminathan L, Kaatz S, Chubb H, Tae K, Ramesh MS, Fadel R, et al. Impact of early corticosteroids on preventing clinical deterioration in non-critically ill patients hospitalized with COVID-19: a multi-hospital cohort study. Infect Dis Ther. 2022; 11(2):887–898. PMID: 35267172.13. Noguchi K, Latif M, Thangavelu K, Konietschke F, Gel YR, Brunner E. nparLD: Nonparametric analysis of longitudinal data in factorial experiments, R package version 2.1. Updated 2012. Accessed October 13, 2022. https://cran.r-project.org/web/packages/nparLD .14. Wang J, Yang W, Chen P, Guo J, Liu R, Wen P, et al. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: a systematic review and meta-analysis. PLoS One. 2021; 16(4):e0249481. PMID: 33882090.15. Becker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesth Prog. 2013; 60(1):25–31. PMID: 23506281.16. Zhan Y, Shang J, Gu Y, Huang Q, Xie J. Efficacy of corticosteroid in patients with COVID-19: a multi-center retrospective study and meta-analysis. J Med Virol. 2021; 93(7):4292–4302. PMID: 33666250.17. Tortajada C, Colomer E, Andreu-Ballester JC, Esparcia A, Oltra C, Flores J. Corticosteroids for COVID-19 patients requiring oxygen support? Yes, but not for everyone: effect of corticosteroids on mortality and intensive care unit admission in patients with COVID-19 according to patients’ oxygen requirements. J Med Virol. 2021; 93(3):1817–1823. PMID: 33107607.18. Keller MJ, Kitsis EA, Arora S, Chen JT, Agarwal S, Ross MJ, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020; 15(8):489–493. PMID: 32804611.19. RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384(8):693–704. PMID: 32678530.20. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020; 8(3):267–276. PMID: 32043986.21. Yang R, Xiong Y, Ke H, Chen T, Gao S. The role of methylprednisolone on preventing disease progression for hospitalized patients with severe COVID-19. Eur J Clin Invest. 2020; 50(11):e13412. PMID: 32954492.22. Pinzón MA, Ortiz S, Holguín H, Betancur JF, Cardona Arango D, Laniado H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One. 2021; 16(5):e0252057. PMID: 34033648.23. Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021; 21(1):337. PMID: 33838657.24. Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J Intensive Care Med. 2021; 36(6):673–680. PMID: 33632000.25. Jamil Z, Almajhdi FN, Khalid S, Asghar M, Ahmed J, Waheed Y. Comparison of low-versus high-dose steroids in the clinical outcome of hospitalized COVID-19 patients. Antibiotics (Basel). 2021; 10(12):1510. PMID: 34943722.26. Monreal E, Sainz de la Maza S, Natera-Villalba E, Beltrán-Corbellini Á, Rodríguez-Jorge F, Fernández-Velasco JI, et al. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2021; 40(4):761–769. PMID: 33083917.27. Chen Y, Li L. Influence of corticosteroid dose on viral shedding duration in patients with COVID-19. Clin Infect Dis. 2021; 72(7):1298–1300. PMID: 32588884.28. Bivona G, Agnello L, Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021; 41(6):540–548. PMID: 34108281.29. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID: 32242348.30. Soliman OM, Moeen SM, Abbas YA, Kamel EZ. The impact of dexamethasone versus methylprednisolone upon neutrophil/lymphocyte ratio in COVID-19 patients admitted to ICU and its implication upon mortality. Egypt J Anaesth. 2022; 38(1):78–84.31. Li S, Hu Z, Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2021; 72(7):1297–1298. PMID: 32588877.32. Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2021; 72(9):e373–e381. PMID: 32785710.33. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020; 324(13):1317–1329. PMID: 32876697.34. van Paassen J, Vos JS, Hoekstra EM, Neumann KM, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020; 24(1):696. PMID: 33317589.35. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020; 324(13):1298–1306. PMID: 32876689.36. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020; 324(13):1307–1316. PMID: 32876695.37. Ritter LA, Britton N, Heil EL, Teeter WA, Murthi SB, Chow JH, et al. The impact of corticosteroids on secondary infection and mortality in critically ill COVID-19 patients. J Intensive Care Med. 2021; 36(10):1201–1208. PMID: 34247526.38. Oh SM, Ham SY, Suh HJ, Lee E, Park SW. Clinical characteristics of COVID-19: use of steroids in mostly unvaccinated COVID-19 patients before the omicron variant. J Korean Med Sci. 2022; 37(29):e228. PMID: 35880504.39. Na YS, Baek AR, Baek MS, Kim WY, Kim JH, Lee BY, et al. Clinical outcomes of and risk factors for secondary infection in patients with severe COVID-19: a multicenter cohort study in South Korea. Korean J Intern Med. 2023; 38(1):68–79. PMID: 36420564.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Methylprednisolone pulse therapy for critically ill patients with COVID-19: a cohort study
- The Effect of the Timing of Dexamethasone Administration in Patients with COVID-19 Pneumonia
- Comparison of critically ill COVID-19 and influenza patients with acute respiratory failure
- New-Onset Seizures in Patients With COVID-19: A Case Series From a Single Public Hospital in Korea
- Outcomes of patients with COVID-19 requiring extracorporeal membrane oxygenation and continuous renal replacement therapy in the United States