Korean J Gastroenterol.
2023 Jul;82(1):10-17. 10.4166/kjg.2023.039.
Efficacy of 7-day Tailored Therapy for Helicobacter pylori Eradication based on Clarithromycin Resistance
- Affiliations
-
- 1Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2544934
- DOI: http://doi.org/10.4166/kjg.2023.039
Abstract
- Background/Aims
Increasing resistance to clarithromycin (CAM) of Helicobacter pylori (H. pylori) is one of the main causes of recent decrease in eradication rate of standard triple therapy. The aim of this study was to evaluate the usefulness of 7-day tailored therapy based on the existence of CAM resistance.
Methods
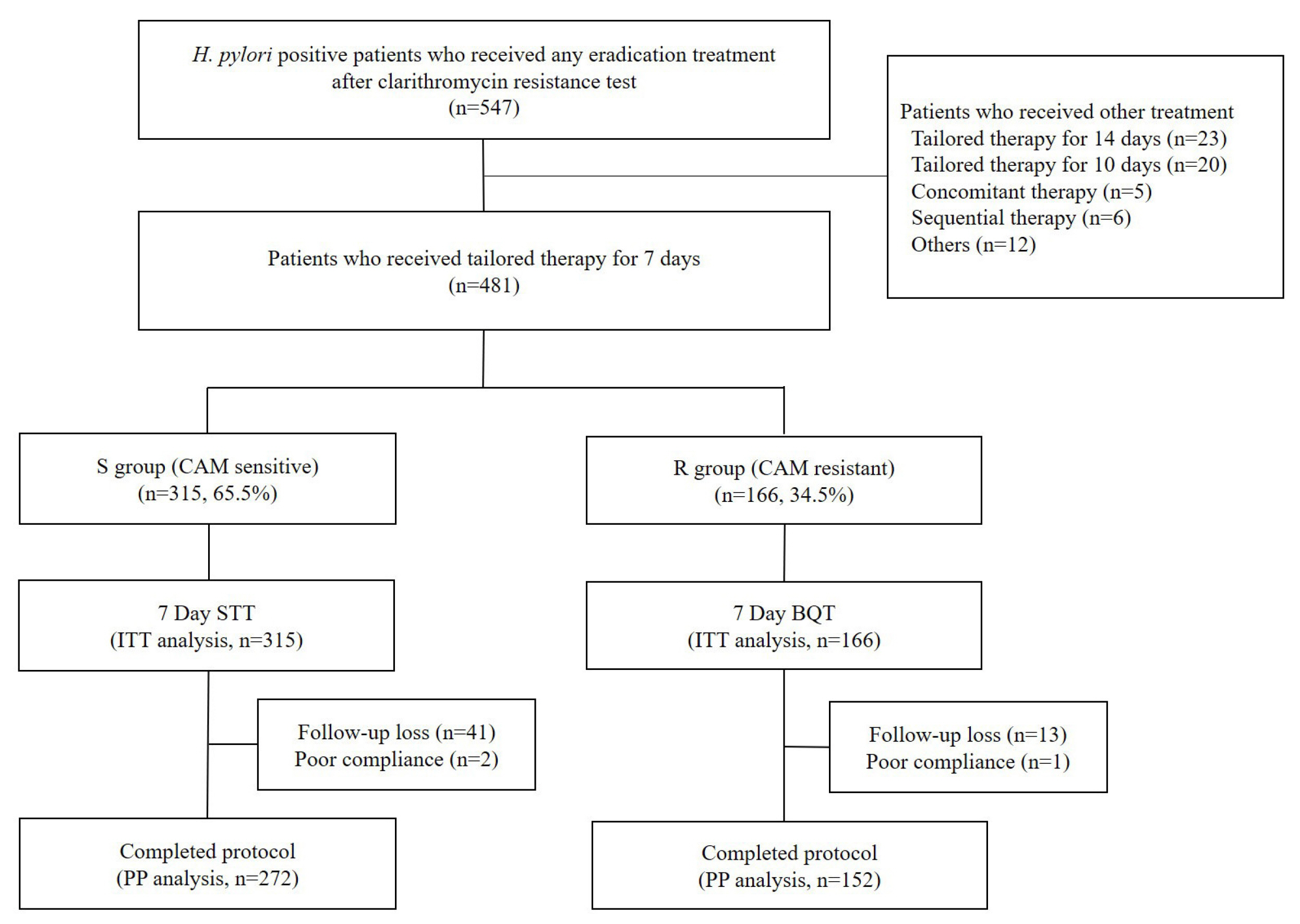
From January 2017 to May 2022, a total of 481 consecutive patients with H. pylori infection were recruited in Daegu Catholic University Medical Center. Treatment regimen was selected based on the result of CAM resistance test. Patients with CAM resistance (R group) were treated with bismuth-based quadruple therapy for 7 days. Patients without CAM resistance (S group) were treated with standard triple therapy for 7 days.
Results
The overall H. pylori eradication rate was 89.4% (379 of 424) by per-protocol (PP) analysis. Patients with CAM resistance mutation included 166 patients (34.5%). The eradication rates of each group were 88.8% (135 of 152) and 89.7% (244 of 272) by PP analysis, for R and S group respectively. By intention-to-treat (ITT) analysis, the eradication rates were 81.3% (135 of 166) and 77.5% (244 of 315) for R and S group. CAM resistance was identified with a dual-priming oligonucleotide-based multiplex PCR.
Conclusions
In spite of this high CAM resistance (34.5%), the eradication rate of 7-day tailored therapy based on the existence of CAM resistance was 89.4%. The 7-day tailored therapy based on CAM resistance could be an acceptable treatment selection strategy for H. pylori eradication.
Figure
Reference
-
1. Malfertheiner P, Megraud F, O'Morain CA, et al. 2017; Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 66:6–30. DOI: 10.1136/gutjnl-2016-312288. PMID: 27707777.2. Fock KM, Graham DY, Malfertheiner P. 2013; Helicobacter pylori research: historical insights and future directions. Nat Rev Gastroenterol Hepatol. 10:495–500. DOI: 10.1038/nrgastro.2013.96. PMID: 23752823. PMCID: PMC3973742.3. Shin SH, Jung DH, Kim JH, et al. 2015; Helicobacter pylori eradication prevents metachronous gastric neoplasms after endoscopic resection of gastric dysplasia. PLoS One. 10:e0143257. DOI: 10.1371/journal.pone.0143257. PMCID: PMC4651354.4. Lee YC, Chiang TH, Chou CK, et al. 2016; Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 150:1113–1124.e5. DOI: 10.1053/j.gastro.2016.01.028. PMID: 26836587.5. Shin WG, Lee SW, Baik GH, et al. 2016; Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: Nationwide survey. Helicobacter. 21:266–278. DOI: 10.1111/hel.12279. PMID: 26470999.6. Kim BJ, Kim HS, Song HJ, et al. 2016; Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: Interim analysis. J Korean Med Sci. 31:1246–1253. DOI: 10.3346/jkms.2016.31.8.1246. PMID: 27478335. PMCID: PMC4951554.7. Gong EJ, Yun SC, Jung HY, et al. 2014; Meta-analysis of first-line triple therapy for Helicobacter pylori eradication in Korea: is it time to change? J Korean Med Sci. 29:704–713. DOI: 10.3346/jkms.2014.29.5.704. PMID: 24851029. PMCID: PMC4024949.8. Kim TH, Park JM, Cheung DY, Oh JH. 2020; Comparison of 7- and 14-day eradication therapy for Helicobacter pylori with first- and second-line regimen: Randomized clinical trial. J Korean Med Sci. 35:e33. DOI: 10.3346/jkms.2020.35.e33. PMID: 32030921. PMCID: PMC7008067.9. Lee JH, Ahn JY, Choi KD, et al. 2019; Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 24:e12592. DOI: 10.1111/hel.12592. PMID: 31111572.10. Park JY, Shin TS, Kim JH, Yoon HJ, Kim BJ, Kim JG. The prevalence of multidrug resistance of Helicobacter pylori and its impact on eradication in Korea from 2017 to 2019: A single-center study. Antibiotics (Basel). 2020; 9:646. DOI: 10.3390/antibiotics9100646. PMID: 32992624.11. Kwon YH, Jeon SW, Nam SY, Lee HS, Park JH. 2019; Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: A single-center prospective pilot study. Helicobacter. 24:e12585. DOI: 10.1111/hel.12585. PMID: 30969459.12. Zullo A, Hassan C, Lorenzetti R, Winn S, Morini S. 2003; A clinical practice viewpoint: to culture or not to culture Helicobacter pylori? Dig Liver Dis. 35:357–361. DOI: 10.1016/S1590-8658(03)00081-1. PMID: 12846409.13. Owen RJ. 2002; Molecular testing for antibiotic resistance in Helicobacter pylori. Gut. 50:285–289. DOI: 10.1136/gut.50.3.285. PMID: 11839700. PMCID: PMC1773144.14. De Francesco V, Margiotta M, Zullo A, et al. 2006; Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 144:94–100. DOI: 10.7326/0003-4819-144-2-200601170-00006. PMID: 16418408.15. Oleastro M, Ménard A, Santos A, et al. 2003; Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 41:397–402. DOI: 10.1128/JCM.41.1.397-402.2003. PMID: 12517879. PMCID: PMC149634.16. Seo SI, Do BJ, Kang JG, et al. 2019; Helicobacter pylori eradication according to sequencing-based 23S ribosomal RNA point mutation associated with clarithromycin resistance. J Clin Med. 9:54. DOI: 10.3390/jcm9010054. PMID: 31881688. PMCID: PMC7019680.17. Ong S, Kim SE, Kim JH, et al. 2019; Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: A multicenter randomized controlled trial. Helicobacter. 24:e12654. DOI: 10.1111/hel.12654. PMID: 31411793.18. Lee HJ, Kim JI, Cheung DY, et al. 2013; Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 208:1123–1130. DOI: 10.1093/infdis/jit287. PMID: 23801607.19. Pan J, Shi Z, Lin D, et al. 2020; Is tailored therapy based on antibiotic susceptibility effective? A multicenter, open-label, randomized trial. Front Med. 14:43–50. DOI: 10.1007/s11684-019-0706-8. PMID: 31907860.20. Choi YI, Chung JW, Park DK, et al. 2019; Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative, open trial. World J Gastroenterol. 25:6743–6751. DOI: 10.3748/wjg.v25.i46.6743. PMID: 31857776.21. Cho JH, Jeon SR, Kim HG, Jin SY, Park S. 2019; Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol. 34:700–706. DOI: 10.1111/jgh.14383. PMID: 30011083.22. Gweon TG, Kim JS, Kim BW. 2018; An Economic modeling study of Helicobacter pylori eradication: Comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. 12:648–654. DOI: 10.5009/gnl18079. PMCID: PMC6254616.23. López-Góngora S, Puig I, Calvet X, et al. 2015; Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 70:2447–2455. DOI: 10.1093/jac/dkv155. PMID: 26078393.24. Jung HK, Kang SJ, Lee YC, et al. 2021; Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Korean J Intern Med. 36:807–838. DOI: 10.3904/kjim.2020.701. PMID: 34092054. PMCID: PMC8273819.25. Seo SI, Lim H, Bang CS, et al. 2022; Bismuth-based quadruple therapy versus metronidazole-intensified triple therapy as a first-line treatment for clarithromycin-resistant Helicobacter pylori infection: A multicenter randomized controlled trial. Gut Liver. 16:697–705. DOI: 10.5009/gnl210365.26. Liou JM, Fang YJ, Chen CC, et al. 2016; Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 388:2355–2365. DOI: 10.1016/S0140-6736(16)31409-X. PMID: 27769562.27. Kim YI, Lee JY, Kim CG, Park B, Park JY, Choi IJ. 2021; Ten-day bismuth-containing quadruple therapy versus 7-day proton pump inhibitor-clarithromycin containing triple therapy as first-line empirical therapy for the Helicobacter pylori infection in Korea: a randomized open-label trial. BMC Gastroenterol. 21:95. DOI: 10.1186/s12876-021-01680-1. PMID: 33653284. PMCID: PMC7923489.28. Na SY, Kim BW, Kim MJ, Choe Y, Kim JS. Effective eradication regimen and duration according to the clarithromycin susceptibility of helicobacter pylori determined using dual priming oligonucleotide-based multiplex polymerase chain reaction. Gut Liver. 2022; Sep. 28. doi: 10.5009/gnl220256. DOI: 10.5009/gnl220256.29. Kim SY, Park JM, Lim CH, et al. 2021; Types of 23S Ribosomal RNA Point Mutations and Therapeutic Outcomes for Helicobacter pylori. Gut Liver. 15:528–536. DOI: 10.5009/gnl20225. PMID: 33376228. PMCID: PMC8283296.30. Woo HY, Park DI, Park H, et al. 2009; Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 14:22–28. DOI: 10.1111/j.1523-5378.2009.00654.x. PMID: 19191892.31. Hwang TJ, Kim N, Kim HB, et al. 2010; Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 44:536–543. DOI: 10.1097/MCG.0b013e3181d04592. PMID: 20179610.32. Park CG, Kim S, Lee EJ, Jeon HS, Han S. 2018; Clinical relevance of point mutations in the 23S rRNA gene in Helicobacter pylori eradication: A prospective, observational study. Medicine (Baltimore). 97:e11835. DOI: 10.1097/MD.0000000000011835. PMID: 30113472. PMCID: PMC6112885.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eradication Therapy for Helicobacter pylori with Diagnostic Test for Clarithromycin Resistance
- Detection of Clarithromycin Resistance in Helicobacter pylori Using the AllplexTM H. pylori & ClariR Assay and the Ezplex® HP-CLA Real-Time PCR Kit
- New Helicobacter pylori Eradication Therapies
- Tailored Therapy Using Bismuth Add-on Standard Triple Therapy vs. Concomitant Therapy: A First-line Regimen for Helicobacter pylori Infection
- Status of Helicobacter pylori Eradication in Japan