Korean J Gastroenterol.
2018 Nov;72(5):237-244. 10.4166/kjg.2018.72.5.237.
New Helicobacter pylori Eradication Therapies
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. jgkimd@cau.ac.kr
- KMID: 2426864
- DOI: http://doi.org/10.4166/kjg.2018.72.5.237
Abstract
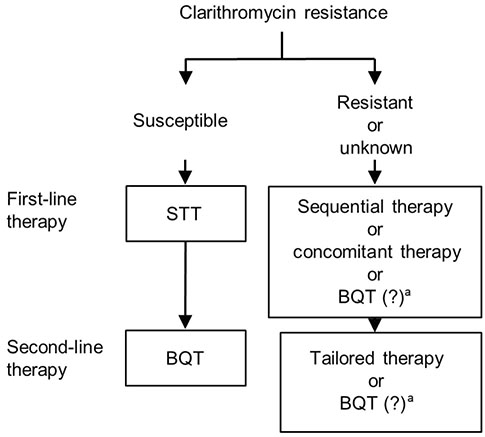
- While the prevalence of Helicobacter pylori (H. pylori) infection is decreasing in Korea, the incidence of gastric cancer remains high, emphasizing the importance of H. pylori eradication. A new treatment strategy is needed as the eradication rate with standard triple therapy, which is currently the standard first-line regimen for H. pylori infection, has decreased below the optimum level. The major cause of eradication failure is increased antibiotic resistance. Sequential, concurrent, and hybrid therapies that include clarithromycin produce higher eradication rates than conventional standard triple therapy. However, the effectiveness of these treatments is limited in regions where the resistance rate to various antibiotics is high. Bismuth quadruple therapy is another alternative therapy, but again the eradication rate is not sufficiently high. Tailored therapy based on individual characteristics, including antibiotic susceptibility, may be ideal, but there are several limitations for clinical application and further research is needed. New potassium-competitive acid blocker-based therapies could emerge as effective alternatives in the near future. A consensus is needed to establish a strategy for applying new eradication therapies in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001; 15:1949–1958.
Article2. Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
Article3. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018; 378:1085–1095.
Article4. Korean H. pylori Study Group. Diagnosis and treatment of Helicobacter pylori infection in Korea. Korean J Gastroenterol. 1998; 32:275–289.5. Kim N, Kim JJ, Choe YH, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.
Article6. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
Article7. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013; 132:1272–1276.
Article8. Ford AC, Forman D, Hunt R, Yuan Y, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev. 2015; (7):CD005583.
Article9. Lee JH, Choi KD, Jung HY, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. 2018; 23:e12463.10. Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016; 21:266–278.11. Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci. 2016; 31:1246–1253.
Article12. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010; 59:1143–1153.
Article13. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017; 112:212–239.
Article14. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017; 66:6–30.15. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific consensus guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.16. Zullo A, Rinaldi V, Winn S, et al. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000; 14:715–718.17. Rinaldi V, Zullo A, Pugliano F, Valente C, Diana F, Attili AF. The management of failed dual or triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 1997; 11:929–933.
Article18. Maconi G, Parente F, Russo A, Vago L, Imbesi V, Bianchi Porro G. Do some patients with Helicobacter pylori infection benefit from an extension to 2 weeks of a proton pump inhibitor-based triple eradication therapy. Am J Gastroenterol? 2001; 96:359–366.
Article19. De Francesco V, Margiotta M, Zullo A, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006; 144:94–100.
Article20. Jung YS, Park CH, Park JH, Nam E, Lee HL. Efficacy of Helicobacter pylori eradication therapies in Korea: a systematic review and network meta-analysis. Helicobacter. 2017; 22:e12389.21. Kim YS, Kim SJ, Yoon JH, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011; 34:1098–1105.
Article22. Choi HS, Chun HJ, Park SH, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012; 18:2377–2382.23. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.24. Lam SK, Talley NJ. Report of the 1997 Asia Pacific consensus conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998; 13:1–12.
Article25. Gatta L, Scarpignato C, Fiorini G, et al. Impact of primary antibiotic resistance on the effectiveness of sequential therapy for Helicobacter pylori infection: lessons from a 5-year study on a large number of strains. Aliment Pharmacol Ther. 2018; 47:1261–1269.
Article26. Gong EJ, Ahn JY. Antimicrobial resistance of Helicobacter pylori isolates in Korea. Korean J Helicobacter Up Gastrointest Res. 2018; 18:82–88.
Article27. Hwang JJ, Lee DH, Yoon H, Shin CM, Park YS, Kim N. Efficacy of moxifloxacin-based sequential and hybrid therapy for first-line Helicobacter pylori eradication. World J Gastroenterol. 2015; 21:10234–10241.28. Hwang JJ, Lee DH, Lee AR, et al. Efficacy of moxifloxacin-based sequential therapy for first-line eradication of Helicobacter pylori infection in gastrointestinal disease. World J Gastroenterol. 2015; 21:5032–5038.29. Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013; 18:180–186.30. Kim SY, Lee SW, Hyun JJ, et al. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple “concomitant” therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013; 47:21–24.
Article31. Chung JW, Han JP, Kim KO, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: a multicenter, prospective study. Dig Liver Dis. 2016; 48:888–892.
Article32. Lee HJ, Kim JI, Lee JS, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015; 21:351–359.33. Lee JY, Ahn JY, Choi IJ. Historical perspective of Helicobacter pylori treatment in Korea. Korean J Helicobacter Up Gastrointest Res. 2015; 15:211–221.34. Gisbert JP, Calvet X, O'Connor A, Mégraud F, O'Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010; 44:313–325.35. Kang BK, Park SM, Kim BW. New therapeutic strategies against Helicobacter pylori. Korean J Gastroenterol. 2014; 63:146–150.36. Park SM, Kim JS, Kim BW, Ji JS, Choi H. Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 2017; 32:589–594.37. Kim SY, Lee SW, Choe JW, et al. Helicobacter pylori eradication rates of concomitant and sequential therapies in Korea. Helicobacter. 2017; 22:e12441.
Article38. Bae HJ, Kim JS, Kim BW, Nam YJ. Concomitant or sequential therapy as the first-line therapy for eradication of Helicobacter pylori infection in Korea: a systematic review and meta-analysis. Korean J Gastroenterol. 2018; 71:31–37.
Article39. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013; 347:f4587.
Article40. He L, Deng T, Luo H. Meta-analysis of sequential, concomitant and hybrid therapy for Helicobacter pylori eradication. Intern Med. 2015; 54:703–710.41. Apostolopoulos P, Koumoutsos I, Ekmektzoglou K, et al. Concomitant versus sequential therapy for the treatment of Helicobacter pylori infection: a Greek randomized prospective study. Scand J Gastroenterol. 2016; 51:145–151.42. Jang HJ, Choi MH, Kim YS, et al. Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2005; 46:368–372.43. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007; 26:343–357.
Article44. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013; (12):CD008337.
Article45. Lee ST, Lee DH, Lim JH, et al. Efficacy of 7-day and 14-day bismuth-containing quadruple therapy and 7-day and 14-day moxifloxacin-based triple therapy as second-line eradication for Helicobacter pylori infection. Gut Liver. 2015; 9:478–485.
Article46. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010; 15:38–45.47. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010; 8:59–70.48. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013; 62:34–42.49. Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011; 16:139–145.50. Hsu PI, Wu DC, Wu JY, Graham DY. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter. 2011; 16:146–152.
Article51. Oh DH, Lee DH, Kang KK, et al. Efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol. 2014; 29:1171–1176.52. Heo J, Jeon SW, Jung JT, et al. Concomitant and hybrid therapy for Helicobacter pylori infection: a randomized clinical trial. J Gastroenterol Hepatol. 2015; 30:1361–1366.53. Garcia N, Calvet X, Gené E, Campo R, Brullet E. Limited usefulness of a seven-day twice-a-day quadruple therapy. Eur J Gastroenterol Hepatol. 2000; 12:1315–1318.
Article54. Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015; 64:1715–1720.55. Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol. 2016; 111:1736–1742.
Article56. Choe JW, Jung SW, Kim SY, et al. Comparative study of Helicobacter pylori eradication rates of concomitant therapy vs modified quadruple therapy comprising proton-pump inhibitor, bismuth, amoxicillin, and metronidazole in Korea. Helicobacter. 2018; 23:e12466.57. Scott DR, Munson KB, Marcus EA, Lambrecht NW, Sachs G. The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+-ATPase. Aliment Pharmacol Ther. 2015; 42:1315–1326.58. Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015; 41:636–648.
Article59. Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015; 42:719–730.60. Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep. 2011; 13:540–546.
Article61. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016; 65:1439–1446.62. Maruyama M, Tanaka N, Kubota D, et al. Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: a randomized controlled trial. Can J Gastroenterol Hepatol. 2017; 2017:4385161.63. Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther. 2017; 46:106–114.64. Lee JY, Kim N. Future trends of Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2014; 63:158–170.65. Lee HJ, Kim JI, Cheung DY, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013; 208:1123–1130.
Article66. Chen H, Dang Y, Zhou X, Liu B, Liu S, Zhang G. Tailored therapy versus empiric chosen treatment for Helicobacter pylori eradication: a meta-analysis. Medicine (Baltimore). 2016; 95:e2750.67. López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015; 70:2447–2455.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Approach to Patients after Successful Eradication of Helicobacter pylori
- Approach to Patients with Consecutive Helicobacter pylori Eradication Failure
- Prevention of Gastric Cancer: Helicobacter pylori Treatment
- Helicobacter pylori Eradication in Patients Undergoing Gastrectomy: Diagnosis and Therapy
- Treatment of Helicobacter pylori infection in functional dyspepsia