Diabetes Metab J.
2023 Jul;47(4):487-499. 10.4093/dmj.2022.0125.
Pharmacologic Activation of Angiotensin-Converting Enzyme II Alleviates Diabetic Cardiomyopathy in db/db Mice by Reducing Reactive Oxidative Stress
- Affiliations
-
- 1Department of Biomedical Science and Engineering, Gwangju Institute of Science and Technology, Gwangju, Korea
- 2Division of Cardiology, Department of Internal Medicine, Inje University Ilsan Paik Hospital, College of Medicine, Inje University, Goyang, Korea
- 3Cardiovascular and Metabolic Disease Center, Smart Marine Therapeutics Center, Inje University, Busan, Korea
- KMID: 2544728
- DOI: http://doi.org/10.4093/dmj.2022.0125
Abstract
- Background
Diabetes mellitus is one of the most common chronic diseases worldwide, and cardiovascular disease is the leading cause of morbidity and mortality in diabetic patients. Diabetic cardiomyopathy (DCM) is a phenomenon characterized by a deterioration in cardiac function and structure, independent of vascular complications. Among many possible causes, the renin-angiotensin-aldosterone system and angiotensin II have been proposed as major drivers of DCM development. In the current study, we aimed to investigate the effects of pharmacological activation of angiotensin-converting enzyme 2 (ACE2) on DCM.
Methods
The ACE2 activator diminazene aceturate (DIZE) was administered intraperitoneally to male db/db mice (8 weeks old) for 8 weeks. Transthoracic echocardiography was used to assess cardiac mass and function in mice. Cardiac structure and fibrotic changes were examined using histology and immunohistochemistry. Gene and protein expression levels were examined using quantitative reverse transcription polymerase chain reaction and Western blotting, respectively. Additionally, RNA sequencing was performed to investigate the underlying mechanisms of the effects of DIZE and identify novel potential therapeutic targets for DCM.
Results
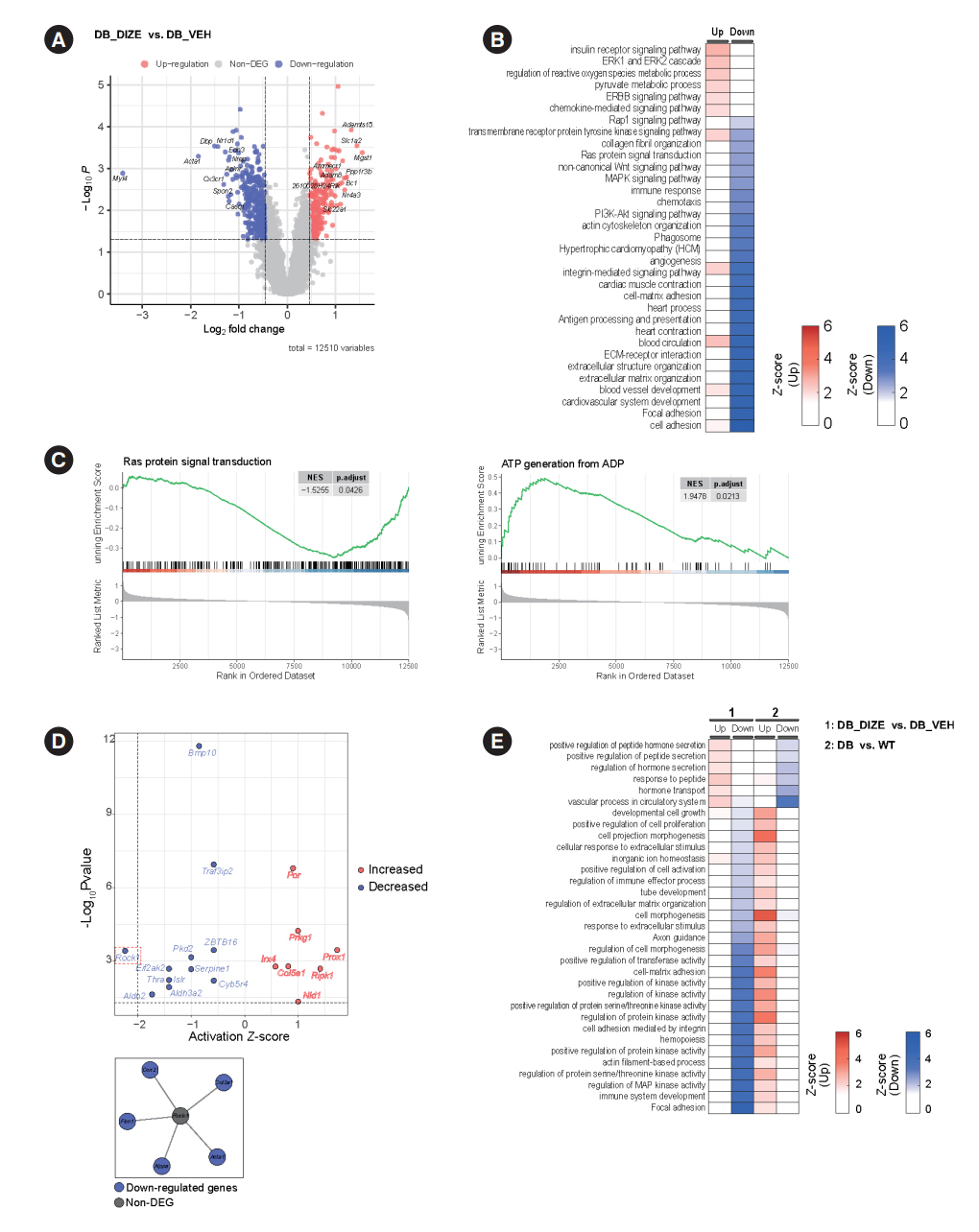
Echocardiography revealed that in DCM, the administration of DIZE significantly improved cardiac function as well as reduced cardiac hypertrophy and fibrosis. Transcriptome analysis revealed that DIZE treatment suppresses oxidative stress and several pathways related to cardiac hypertrophy.
Conclusion
DIZE prevented the diabetes mellitus-mediated structural and functional deterioration of mouse hearts. Our findings suggest that the pharmacological activation of ACE2 could be a novel treatment strategy for DCM.
Keyword
Figure
Reference
-
1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018; 138:271–81.
Article2. Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019; 8:e011295.
Article3. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018; 122:624–38.4. Sun L, Yu M, Zhou T, Zhang S, He G, Wang G, et al. Current advances in the study of diabetic cardiomyopathy: from clinicopathological features to molecular therapeutics (Review). Mol Med Rep. 2019; 20:2051–62.
Article5. Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, et al. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001; 50:2363–75.
Article6. Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010; 11:31–9.
Article7. Zhang F, Liu J, Li SF, Song JX, Ren JY, Chen H. Angiotensin-(1-7): new perspectives in atherosclerosis treatment. J Geriatr Cardiol. 2015; 12:676–82.8. Harvey B, Jaisser F. Aldosterone-mineralocorticoid receptor: cell biology to translational medicine. IntechOpen;2019. Chapter 5, Renin-angiotensin-aldosterone system in heart failure: focus on nonclassical angiotensin pathways as novel upstream targets regulating aldosterone [cited 2022 Jul 12]. Available from:https://doi.org/10.5772/intechopen.87239.
Article9. Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, et al. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008; 51:712–8.
Article10. Zhao YX, Yin HQ, Yu QT, Qiao Y, Dai HY, Zhang MX, et al. ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum Gene Ther. 2010; 21:1545–54.
Article11. Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, et al. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res. 2012; 110:1322–35.
Article12. Dong B, Yu QT, Dai HY, Gao YY, Zhou ZL, Zhang L, et al. Angiotensin-converting enzyme-2 overexpression improves left ventricular remodeling and function in a rat model of diabetic cardiomyopathy. J Am Coll Cardiol. 2012; 59:739–47.
Article13. Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, et al. Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail. 2014; 7:327–39.14. Qaradakhi T, Gadanec LK, McSweeney KR, Tacey A, Apostolopoulos V, Levinger I, et al. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin Exp Pharmacol Physiol. 2020; 47:751–8.
Article15. Evans CE, Miners JS, Piva G, Willis CL, Heard DM, Kidd EJ, et al. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020; 139:485–502.
Article16. Goru SK, Kadakol A, Malek V, Pandey A, Sharma N, Gaikwad AB. Diminazene aceturate prevents nephropathy by increasing glomerular ACE2 and AT2 receptor expression in a rat model of type1 diabetes. Br J Pharmacol. 2017; 174:3118–30.17. Duan R, Xue X, Zhang QQ, Wang SY, Gong PY, Yan E, et al. ACE2 activator diminazene aceturate ameliorates Alzheimer’s disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging (Albany NY). 2020; 12:14819–29.
Article18. Kamel AS, Abdelkader NF, Abd El-Rahman SS, Emara M, Zaki HF, Khattab MM. Stimulation of ACE2/ANG(1-7)/Mas axis by diminazene ameliorates Alzheimer’s disease in the D-galactose-ovariectomized rat model: role of PI3K/Akt pathway. Mol Neurobiol. 2018; 55:8188–202.
Article19. Oh CM, Cho S, Jang JY, Kim H, Chun S, Choi M, et al. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J. 2019; 49:1183–95.
Article20. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010; 26:139–40.21. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4:44–57.
Article22. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005; 102:15545–50.
Article23. Morris JH, Apeltsin L, Newman AM, Baumbach J, Wittkop T, Su G, et al. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinformatics. 2011; 12:436.
Article24. Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945; 26:297–302.
Article25. Mueller LN, Rinner O, Schmidt A, Letarte S, Bodenmiller B, Brusniak MY, et al. SuperHirn: a novel tool for high resolution LC-MS-based peptide/protein profiling. Proteomics. 2007; 7:3470–80.
Article26. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–504.
Article27. Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014; 30:523–30.
Article28. Sergeeva IA, Christoffels VM. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochim Biophys Acta. 2013; 1832:2403–13.
Article29. Rajapaksha IG, Mak KY, Huang P, Burrell LM, Angus PW, Herath CB. The small molecule drug diminazene aceturate inhibits liver injury and biliary fibrosis in mice. Sci Rep. 2018; 8:10175.
Article30. Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig. 2014; 5:623–34.
Article31. Ramos-Kuri M, Meka SH, Salamanca-Buentello F, Hajjar RJ, Lipskaia L, Chemaly ER. Molecules linked to Ras signaling as therapeutic targets in cardiac pathologies. Biol Res. 2021; 54:23.
Article32. Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009; 81:412–9.
Article33. Huang X, Zhang KJ, Jiang JJ, Jiang SY, Lin JB, Lou YJ. Identification of crucial genes and key functions in type 2 diabetic hearts by bioinformatic analysis. Front Endocrinol (Lausanne). 2022; 13:801260.
Article34. Morimoto H, Mori J, Nakajima H, Kawabe Y, Tsuma Y, Fukuhara S, et al. Angiotensin 1-7 stimulates brown adipose tissue and reduces diet-induced obesity. Am J Physiol Endocrinol Metab. 2018; 314:E131–8.
Article35. Kawabe Y, Mori J, Morimoto H, Yamaguchi M, Miyagaki S, Ota T, et al. ACE2 exerts anti-obesity effect via stimulating brown adipose tissue and induction of browning in white adipose tissue. Am J Physiol Endocrinol Metab. 2019; 317:E1140–9.
Article36. Velkoska E, Patel SK, Griggs K, Burrell LM. Diminazene aceturate improves cardiac fibrosis and diastolic dysfunction in rats with kidney disease. PLoS One. 2016; 11:e0161760.
Article37. Rabelo LA, Alenina N, Bader M. ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res. 2011; 34:154–60.
Article38. Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001; 1:181–93.
Article39. Morgenstern R, Zhang J, Johansson K. Microsomal glutathione transferase 1: mechanism and functional roles. Drug Metab Rev. 2011; 43:300–6.
Article40. Kuang F, Liu J, Xie Y, Tang D, Kang R. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem Biol. 2021; 28:765–75.
Article41. Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021; 11:3052–9.
Article42. Hartmann S, Ridley AJ, Lutz S. The function of Rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol. 2015; 6:276.
Article43. Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006; 20:916–25.
Article44. Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/- haploinsufficient mice. Circulation. 2005; 112:2959–65.45. Shimizu T, Liao JK. Rho kinases and cardiac remodeling. Circ J. 2016; 80:1491–8.46. Guan P, Liang Y, Wang N. Fasudil alleviates pressure overload-induced heart failure by activating Nrf2-mediated antioxidant responses. J Cell Biochem. 2018; 119:6452–60.47. Dong LY, Qiu XX, Zhuang Y, Xue S. Y-27632, a Rho-kinase inhibitor, attenuates myocardial ischemia-reperfusion injury in rats. Int J Mol Med. 2019; 43:1911–9.48. Fraga-Silva RA, Sorg BS, Wankhede M, Dedeugd C, Jun JY, Baker MB, et al. ACE2 activation promotes antithrombotic activity. Mol Med. 2010; 16:210–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Betaine Alleviates Hypertriglycemia and Tau Hyperphosphorylation in db/db Mice
- Effect of glucagon-like peptide 1 on salivary gland hypofunction in diabetic db/db mice
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- The Role of Oxidative Stress in the Pathogenesis of Diabetic Vascular Complications
- Effect of Angiotensin Converting Enzyme Inhibitors on Induced Angiotensin Converting Enzyme Activity in Rat Intestine