J Rhinol.
2023 Jul;30(2):62-68. 10.18787/jr.2023.00029.
Effectiveness of Dupilumab Treatment to Treat Chronic Rhinosinusitis With Nasal Polyposis: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 2Department of Otolaryngology-Head and Neck Surgery, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- KMID: 2544615
- DOI: http://doi.org/10.18787/jr.2023.00029
Abstract
- Background and Objectives
Evidence bearing on the safety and efficacy of dupilumab treatment for chronic rhinosinusitis with nasal polyps (CRSwNP) has recently been presented by researchers from various institutions. Therefore, we compared the safety and efficacy of dupilumab treatment to those of endoscopic sinus surgery.
Methods
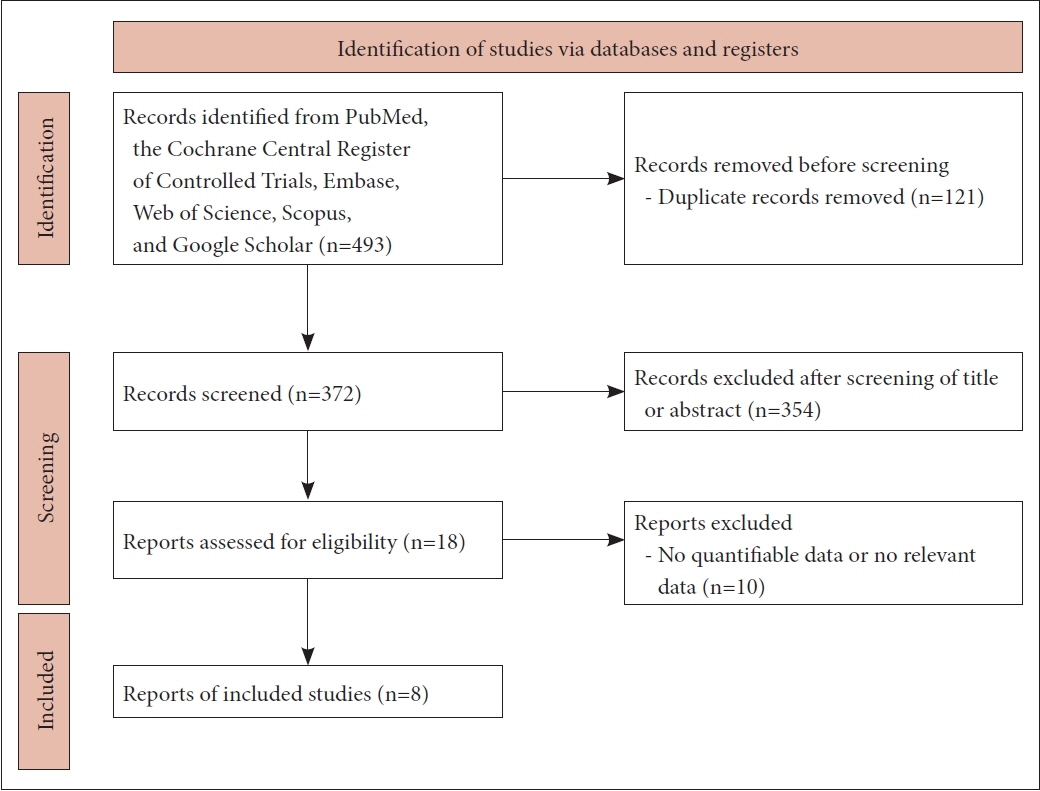
The PubMed, Scopus, Embase, Web of Science, and Cochrane databases were searched independently by two authors from the dates of their inception to December 2022. We retrieved the clinical results of CRSwNP patients after dupilumab administration, including changes in patient symptoms and the effects on the quality of life, and compared the results of dupilumab (treatment group) to those of endoscopic sinus surgery (control group).
Results
Eight articles (1,251 patients) were ultimately included. Dupilumab significantly improved nasal symptoms (nasal congestion) (mean difference [MD], -1.4433; 95% confidence interval [CI], -1.7233 to -1.1632; I2=94.2%), the visual analog sinusitis score (MD, -5.0506; 95% CI, -5.4744 to -4.6267; I2=84.0%), olfactory function (standardized MD, 1.2691; 95% CI, 1.1549 to 1.3833; I2=18.4%), the quality of life (SNOT-22 score) (MD, -34.4941; 95% CI, -39.4187 to -29.5695; I2=90.8%), the Lund-Mackay computed tomography score (MD, -7.2713; 95% CI, -8.9442 to -5.5984; I2=87.7%), and the nasal polyp score (MD, -3.1021; 95% CI, -3.7066 to -2.4977; I2=95.6%) at about 12 months after treatment compared to the pretreatment values. Compared to endoscopic sinus surgery, dupilumab similarly improved olfactory function (MD, 1.9849; 95% CI, -1.6190 to 5.5888; I2=0.0%) but was less effective in terms of reducing the SNOT-22 score (MD, 3.8472; 95% CI, 1.9872 to 5.7073; I2=96.7%) and reducing nasal congestion (MD, 0.6519; 95% CI, 0.5619 to 0.7420; I2=97.7%).
Conclusion
Dupilumab reduced subjective symptom scores and improved the quality of life and objective measures of progression compared to the preoperative values.
Figure
Cited by 1 articles
-
Biologics for Chronic Rhinosinusitis With Nasal Polyps: Current Status and Clinical Considerations in Korea
Ki-Il Lee, Gwanghui Ryu, Shin Hyuk Yoo, Hyung-Ju Cho, Ji-Hun Mo, Chang-Hoon Kim
J Rhinol. 2025;32(1):1-9. doi: 10.18787/jr.2025.00002.
Reference
-
References
1. Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin Immunol. 1977; 59(1):17–21.2. Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999; 28(4):717–22.3. Zhang Y, Gevaert E, Lou H, Wang X, Zhang L, Bachert C, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017; 140(5):1230–9.4. Bachert C, Hellings PW, Mullol J, Hamilos DL, Gevaert P, Naclerio RM, et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2020; 75(1):148–57.5. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; 58(Suppl S29):1–464.6. Trimarchi M, Indelicato P, Vinciguerra A, Bussi M. Clinical efficacy of dupilumab in the treatment of severe chronic rhinosinusitis: the first case outside of a clinical trial. Clin Case Rep. 2021; 9(3):1428–32.7. Patel GB, Peters AT. The role of biologics in chronic rhinosinusitis with nasal polyps. Ear Nose Throat J. 2021; 100(1):44–7.8. Fujieda S, Matsune S, Takeno S, Asako M, Takeuchi M, Fujita H, et al. The effect of dupilumab on intractable chronic rhinosinusitis with nasal polyps in Japan. Laryngoscope. 2021; 131(6):E1770–7.9. Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019; 394(10209):1638–50.10. Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022; 15(3):230–46.11. Hwang SH, Kim JS, Choi BY, Kim JK, Kim BG. Practical review of olfactory training and COVID-19. J Rhinol. 2022; 29(3):127–33.12. Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016; 315(5):469–79.13. Bertlich M, Freytag S, Dombrowski T, Jurmeister P, Spiegel JL, Bertlich I, et al. Subgroups in the treatment of nasal polyposis with dupilumab: a retrospective study. Medicine (Baltimore). 2022; 101(45):e31031.14. Dharmarajan H, Falade O, Lee SE, Wang EW. Outcomes of dupilumab treatment versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2022; 12(8):986–95.15. Miglani A, Soler ZM, Smith TL, Mace JC, Schlosser RJ. A comparative analysis of endoscopic sinus surgery versus biologics for treatment of chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2023; 13(2):116–28.16. Haxel BR, Hummel T, Fruth K, Lorenz K, Gunder N, Nahrath P, et al. Real-world-effectiveness of biological treatment for severe chronic rhinosinusitis with nasal polyps. Rhinology. 2022; 60(6):435.17. Torretta S, De Corso E, Nava N, Fraccaroli F, Ferrucci SM, Settimi S, et al. Proposal for a structured outpatient clinic for dupilumab treatment in chronic rhinosinusitis with nasal polyps in the first year of treatment. J Pers Med. 2022; 12(10):1734.18. Matsuyama T, Takahashi H, Tada H, Chikamatsu K. Circulating T cell subsets and ILC2s are altered in patients with chronic rhinosinusitis with nasal polyps after dupilumab treatment. Am J Rhinol Allergy. 2023; 37(1):58–64.19. Bachert C, Akdis CA. Phenotypes and emerging endotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016; 4(4):621–8.20. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137(5):1449–56.e4.21. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138(5):1344–53.22. Orlandi RR, Kingdom TT, Smith TL, Bleier B, DeConde A, Luong AU, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021; 11(3):213–739.23. Wynn R, Har-El G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2004; 114(5):811–3.24. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017; 127(3):550–5.25. Laidlaw TM, Buchheit KM. Biologics in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol. 2020; 124(4):326–32.26. Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019; 143(1):1–11.27. Bakakos A, Loukides S, Usmani OS, Bakakos P. Biologics in severe asthma: the overlap endotype - opportunities and challenges. Expert Opin Biol Ther. 2020; 20(12):1427–34.28. Kuznik A, Bégo-Le-Bagousse G, Eckert L, Gadkari A, Simpson E, Graham CN, et al. Economic evaluation of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Dermatol Ther (Heidelb). 2017; 7(4):493–505.29. Brown WC, Senior B. A critical look at the efficacy and costs of biologic therapy for chronic rhinosinusitis with nasal polyposis. Curr Allergy Asthma Rep. 2020; 20(6):16.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Events of Dupilumab Injection in Patients With Chronic Rhinosinusitis With Nasal Polyposis
- Update on Biologics in Treatment of Chronic Rhinosinusitis With Nasal Polyposis
- Efficacy and Safety of Dupilumab for Chronic Rhinosinusitis With Nasal Polyps: A Retrospective Study of Every Month Injection
- Practical Review of Biologics in Chronic Rhinosinusitis With Nasal Polyps
- The Role of Superantigen in Nasal Polypogenesis