Korean J Pain.
2023 Jul;36(3):281-298. 10.3344/kjp.23129.
Antimicrobial therapies for chronic pain (part 1): analgesic mechanisms
- Affiliations
-
- 1Department of Anesthesiology and Critical Care Medicine, Division of Pain Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 2Departments of Orthopedic Surgery and Anesthesiology, University of Maryland School of Medicine, Baltimore, MD, USA
- 3Department of Anesthesiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 4Department of Physical Medicine and Rehabilitation, Walter Reed National Military Medical Center, Bethesda, MD, USA
- 5Departments of Physical Medicine & Rehabilitation, Neurology, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 6Departments of Physical Medicine & Rehabilitation and Anesthesiology, Walter Reed National Military Medical Center, Uniformed Services University of the Health Sciences, Bethesda, MD, USA
- KMID: 2544241
- DOI: http://doi.org/10.3344/kjp.23129
Abstract
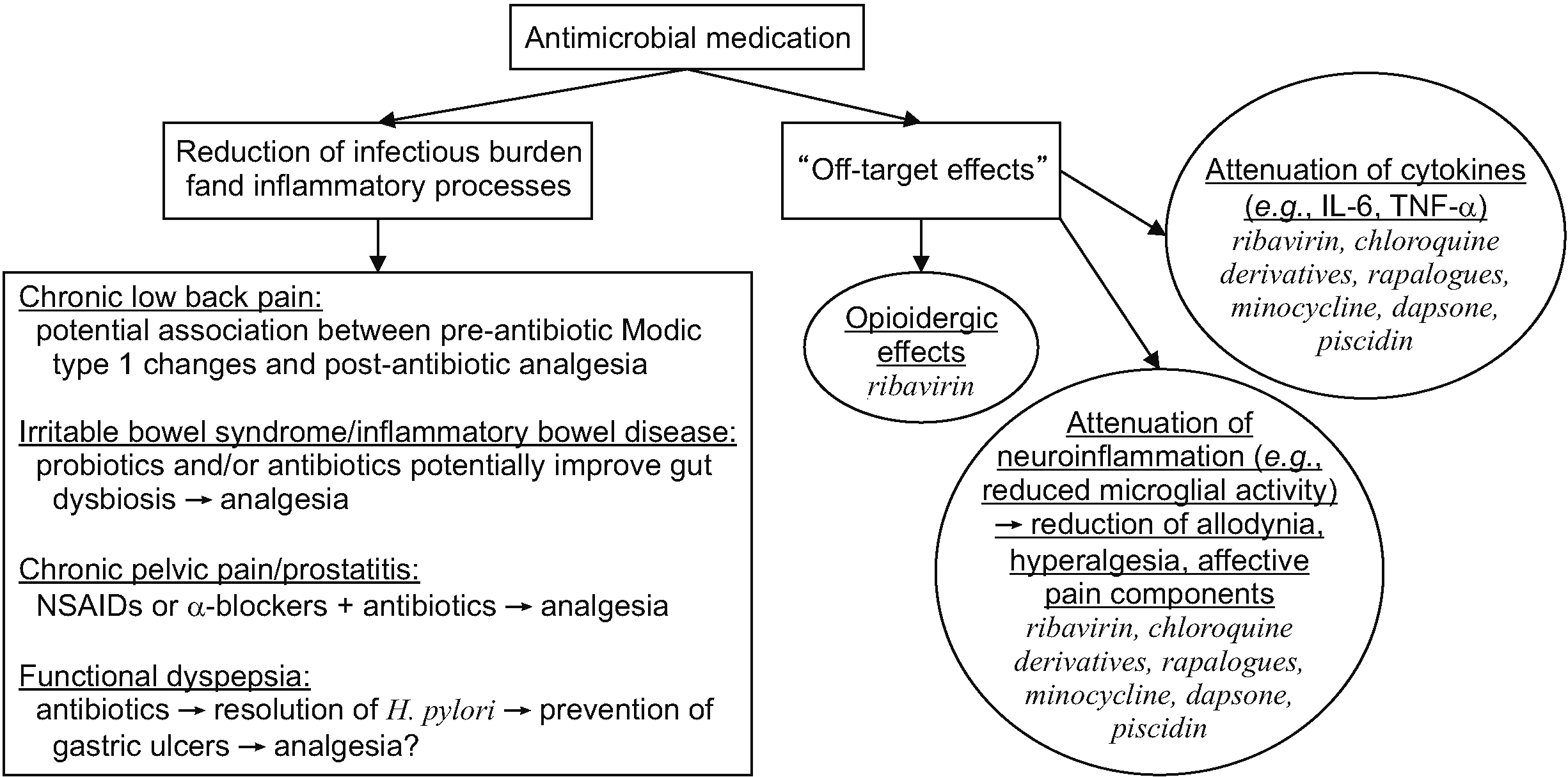
- There is increasing evidence that the relationship between chronic pain and infections is complex and intertwined. Bacterial and viral infections can cause pain through numerous mechanisms such as direct tissue damage and inflammation, the induction of excessive immunologic activity, and the development of peripheral or central sensitization. Treating infections might relieve pain by attenuating these processes, but a growing body of literature suggests that some antimicrobial therapies confer analgesic effects, including for nociceptive and neuropathic pain symptoms, and affective components of pain. The analgesic mechanisms of antimicrobials are indirect, but might be conceptualized into two broad categories: 1) the reduction of the infectious burden and associated pro-inflammatory processes; and 2) the inhibition of signaling processes (e.g., enzymatic and cytokine activity) necessary for nociception and maladaptive neuroplastic changes via off-target effects (unintended binding sites). For the former, there is evidence that symptoms of chronic low back pain (when associated with Modic type 1 changes), irritable bowel syndrome, inflammatory bowel disease, chronic pelvic pain, and functional dyspepsia might be improved after antibiotic treatment, though significant questions remain regarding specific regimens and dose, and which subpopulations are most likely to benefit. For the latter, there is evidence that several antimicrobial classes and medications exert analgesic effects independent of their reduction of infectious burden, and these include cephalosporins, ribavirin, chloroquine derivatives, rapalogues, minocycline, dapsone, and piscidin-1. This article aims to comprehensively review the existing literature for antimicrobial agents that have demonstrated analgesic efficacy in preclinical or clinical studies.
Keyword
Figure
Reference
-
1. Cohen SP, Wang EJ, Doshi TL, Vase L, Cawcutt KA, Tontisirin N. 2022; Chronic pain and infection: mechanisms, causes, conditions, treatments, and controversies. BMJ Med. 1:e000108. DOI: 10.1136/bmjmed-2021-000108. PMID: 36936554. PMCID: PMC10012866.
Article2. Brizzi KT, Lyons JL. 2014; Peripheral nervous system manifestations of infectious diseases. Neurohospitalist. 4:230–40. DOI: 10.1177/1941874414535215. PMID: 25360209. PMCID: PMC4212417.
Article3. Kramer S, Baeumler P, Geber C, Fleckenstein J, Simang M, Haas L, et al. 2019; Somatosensory profiles in acute herpes zoster and predictors of postherpetic neuralgia. Pain. 160:882–94. DOI: 10.1097/j.pain.0000000000001467. PMID: 30585985.
Article4. Gilligan CJ, Cohen SP, Fischetti VA, Hirsch JA, Czaplewski LG. 2021; Chronic low back pain, bacterial infection and treatment with antibiotics. Spine J. 21:903–14. DOI: 10.1016/j.spinee.2021.02.013. PMID: 33610802.
Article5. Cai Z, Zhu T, Liu F, Zhuang Z, Zhao L. 2021; Co-pathogens in periodontitis and inflammatory bowel disease. Front Med (Lausanne). 8:723719. DOI: 10.3389/fmed.2021.723719. PMID: 34616755. PMCID: PMC8488124. PMID: b651136bdaec4a04808c734b2be38365.
Article6. Minerbi A, Shen S. 2022; Gut microbiome in anesthesiology and pain medicine. Anesthesiology. 137:93–108. DOI: 10.1097/ALN.0000000000004204. PMID: 35486831. PMCID: PMC9183187.
Article7. Song JH. 2003; Introduction: the goals of antimicrobial therapy. Int J Infect Dis. 7 Suppl 1:S1–4. DOI: 10.1016/S1201-9712(03)90064-6. PMID: 12839701.
Article8. Gilbert DN, Chambers HF, Saag MS, Pavia AT, Boucher HW.9. Crockett MT, Kelly BS, van Baarsel S, Kavanagh EC. 2017; Modic type 1 vertebral endplate changes: injury, inflammation, or infection? AJR Am J Roentgenol. 209:167–70. DOI: 10.2214/AJR.16.17403. PMID: 28402132.
Article10. Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, et al. 2013; Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J. 22:690–6. DOI: 10.1007/s00586-013-2674-z. PMID: 23397187. PMCID: PMC3631023.
Article11. Albert HB, Sorensen JS, Christensen BS, Manniche C. 2013; Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 22:697–707. DOI: 10.1007/s00586-013-2675-y. PMID: 23404353. PMCID: PMC3631045.
Article12. Bråten LCH, Rolfsen MP, Espeland A, Wigemyr M, Aßmus J, Froholdt A, et al. AIM study group. 2019; Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): double blind, randomised, placebo controlled, multicentre trial. BMJ. 367:l5654. DOI: 10.1136/bmj.l5654. PMID: 31619437. PMCID: PMC6812614.
Article13. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. 2018; Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 48:1044–60. DOI: 10.1111/apt.15001. PMID: 30294792.
Article14. Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. 2017; Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther. 46:115–25. DOI: 10.1111/apt.14108. PMID: 28470846.
Article15. Castiglione F, Rispo A, Di Girolamo E, Cozzolino A, Manguso F, Grassia R, et al. 2003; Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn's disease. Aliment Pharmacol Ther. 18:1107–12. DOI: 10.1046/j.1365-2036.2003.01800.x. PMID: 14653830.
Article16. Anothaisintawee T, Attia J, Nickel JC, Thammakraisorn S, Numthavaj P, McEvoy M, et al. 2011; Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA. 305:78–86. DOI: 10.1001/jama.2010.1913. PMID: 21205969.
Article17. Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. 2016; Helicobacter pylori eradication therapy for functional dyspepsia: systematic review and meta-analysis. World J Gastroenterol. 22:3486–95. DOI: 10.3748/wjg.v22.i12.3486. PMID: 27022230. PMCID: PMC4806206.18. Tan VP, Liu KS, Lam FY, Hung IF, Yuen MF, Leung WK. 2017; Randomised clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Aliment Pharmacol Ther. 45:767–76. DOI: 10.1111/apt.13945. PMID: 28112426.
Article19. Ford AC, Mahadeva S, Carbone MF, Lacy BE, Talley NJ. 2020; Functional dyspepsia. Lancet. 396:1689–702. DOI: 10.1016/S0140-6736(20)30469-4. PMID: 33049222.
Article20. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. faculty members of Kyoto Global Consensus Conference. 2015; Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 64:1353–67. DOI: 10.1136/gutjnl-2015-309252. PMID: 26187502. PMCID: PMC4552923.
Article21. National Cancer Institute. 2011. NCI dictionary of cancer terms [Internet]. National Cancer Institute;Bethesda (MD): https://www.cancer.gov/publications/dictionaries/cancer-terms.22. Bennett JE, Dolin R, Blaser MJ. 2015. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed. Saunders;p. 278–92.e4. DOI: 10.1016/c2012-1-00075-6.23. Bui T, Preuss CV. 2023. Cephalosporins. StatPearls [Internet]. StatPearls Publishing.
Article24. Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. 2005; Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 433:73–7. DOI: 10.1038/nature03180. PMID: 15635412.
Article25. Hu Y, Li W, Lu L, Cai J, Xian X, Zhang M, et al. 2010; An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain. 148:284–301. DOI: 10.1016/j.pain.2009.11.014. PMID: 20022427.
Article26. Hajhashemi V, Hosseinzadeh H, Amin B. 2013; Antiallodynia and antihyperalgesia effects of ceftriaxone in treatment of chronic neuropathic pain in rats. Acta Neuropsychiatr. 25:27–32. DOI: 10.1111/j.1601-5215.2012.00656.x. PMID: 26953071.
Article27. Amin B, Hajhashemi V, Hosseinzadeh H. Abnous Kh. 2012; Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience. 224:15–25. DOI: 10.1016/j.neuroscience.2012.07.058. PMID: 22871519.
Article28. Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, et al. 2007; Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 38:177–82. DOI: 10.1161/01.STR.0000252091.36912.65. PMID: 17122424.
Article29. Mohan A, Lefstein KM, Chang E. 2021; Minocycline and cephalexin in a patient with spastic neuropathic pain secondary to neurosarcoidosis. Pain Med. 22:2767–79. DOI: 10.1093/pm/pnab044. PMID: 33560414.
Article30. Macaluso A, Bernabucci M, Trabucco A, Ciolli L, Troisi F, Baldini R, et al. 2013; Analgesic effect of a single preoperative dose of the antibiotic ceftriaxone in humans. J Pain. 14:604–12. DOI: 10.1016/j.jpain.2013.01.774. PMID: 23725677.
Article31. Rao PS, Goodwani S, Bell RL, Wei Y, Boddu SH, Sari Y. 2015; Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience. 295:164–74. DOI: 10.1016/j.neuroscience.2015.03.038. PMID: 25813713. PMCID: PMC4408259.
Article32. Loustaud-Ratti V, Debette-Gratien M, Jacques J, Alain S, Marquet P, Sautereau D, et al. 2016; Ribavirin: past, present and future. World J Hepatol. 8:123–30. DOI: 10.4254/wjh.v8.i2.123. PMID: 26807208. PMCID: PMC4716528.
Article33. Dixit NM, Perelson AS. 2006; The metabolism, pharmacokinetics and mechanisms of antiviral activity of ribavirin against hepatitis C virus. Cell Mol Life Sci. 63:832–42. DOI: 10.1007/s00018-005-5455-y. PMID: 16501888.
Article34. Abdel-Salam OM. 2006; Antinociceptive and behavioral effects of ribavirin in mice. Pharmacol Biochem Behav. 83:230–8. DOI: 10.1016/j.pbb.2006.01.010. PMID: 16563475.
Article35. Milicevic I, Pekovic S, Subasic S, Mostarica-Stojkovic M, Stosic-Grujicic S, Medic-Mijacevic L, et al. 2003; Ribavirin reduces clinical signs and pathological changes of experimental autoimmune encephalomyelitis in Dark Agouti rats. J Neurosci Res. 72:268–78. DOI: 10.1002/jnr.10552. PMID: 12672002.
Article36. Lavrnja I, Savic D, Bjelobaba I, Dacic S, Bozic I, Parabucki A, et al. 2012; The effect of ribavirin on reactive astrogliosis in experimental autoimmune encephalomyelitis. J Pharmacol Sci. 119:221–32. DOI: 10.1254/jphs.12004FP. PMID: 22785017.
Article37. Liao SH, Li Y, Lai YN, Liu N, Zhang FX, Xu PP. 2017; Ribavirin attenuates the respiratory immune responses to influenza viral infection in mice. Arch Virol. 162:1661–9. DOI: 10.1007/s00705-017-3291-7. PMID: 28243801.
Article38. Romeo-Guitart D, Casas C. 2020; NeuroHeal treatment alleviates neuropathic pain and enhances sensory axon regeneration. Cells. 9:808. DOI: 10.3390/cells9040808. PMID: 32230770. PMCID: PMC7226810.
Article39. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019; 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 78:736–45. DOI: 10.1136/annrheumdis-2019-215089. PMID: 30926722.
Article40. Rempenault C, Combe B, Barnetche T, Gaujoux-Viala C, Lukas C, Morel J, et al. 2020; Clinical and structural efficacy of hydroxychloroquine in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken). 72:36–40. DOI: 10.1002/acr.23826. PMID: 30629341.
Article41. Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. 2023; EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 82:3–18. Erratum in: Ann Rheum Dis 2023; 82: e76. DOI: 10.1136/ard-2022-223356corr1. PMID: 36764818.42. inivasa A Sr, Tosounidou S, Gordon C. 2017; Increased incidence of gastrointestinal side effects in patients taking hydroxychloroquine: a brand-related issue? J Rheumatol. 44:398. DOI: 10.3899/jrheum.161063. PMID: 28250164.
Article43. Khosa S, Khanlou N, Khosa GS, Mishra SK. 2018; Hydroxychloroquine-induced autophagic vacuolar myopathy with mitochondrial abnormalities. Neuropathology. 38:646–52. DOI: 10.1111/neup.12520. PMID: 30411412.
Article44. Jorge A, Ung C, Young LH, Melles RB, Choi HK. 2018; Hydroxychloroquine retinopathy - implications of research advances for rheumatology care. Nat Rev Rheumatol. 14:693–703. DOI: 10.1038/s41584-018-0111-8. PMID: 30401979.
Article45. Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. 2018; Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 14:1435–55. DOI: 10.1080/15548627.2018.1474314. PMID: 29940786. PMCID: PMC6103682.
Article46. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. 2011; Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 186:4794–804. DOI: 10.4049/jimmunol.1000702. PMID: 21398612.
Article47. Jang CH, Choi JH, Byun MS, Jue DM. 2006; Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford). 45:703–10. DOI: 10.1093/rheumatology/kei282. PMID: 16418198.48. Wu SF, Chang CB, Hsu JM, Lu MC, Lai NS, Li C, et al. 2017; Hydroxychloroquine inhibits CD154 expression in CD4+ T lymphocytes of systemic lupus erythematosus through NFAT, but not STAT5, signaling. Arthritis Res Ther. 19:183. DOI: 10.1186/s13075-017-1393-y. PMID: 28793932. PMCID: PMC5550984. PMID: 5482f95b17cb4423a7109e8d1c88b93e.
Article49. Schrezenmeier E, Dörner T. 2020; Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 16:155–66. DOI: 10.1038/s41584-020-0372-x. PMID: 32034323.
Article50. Faraone I, Labanca F, Ponticelli M, De Tommasi N, Milella L. 2020; Recent clinical and preclinical studies of hydroxychloroquine on RNA viruses and chronic diseases: a systematic review. Molecules. 25:5318. DOI: 10.3390/molecules25225318. PMID: 33202656. PMCID: PMC7696151. PMID: c057ffbf9b714013a9dfccc8daab1f80.
Article51. Chou AK, Chiu CC, Wang JJ, Chen YW, Hung CH. 2021; Antimalarial primaquine for spinal sensory and motor blockade in rats. J Pharm Pharmacol. 73:1513–9. DOI: 10.1093/jpp/rgab054. PMID: 34370863.
Article52. Chang YJ, Liu KS, Wang JJ, Hung CH, Chen YW. 2020; Chloroquine for prolonged skin analgesia in rats. Neurosci Lett. 735:135233. DOI: 10.1016/j.neulet.2020.135233. PMID: 32622927.
Article53. Chang YJ, Liu KS, Wang JJ, Chen YW, Hung CH. 2021; Antimalarial primaquine for skin infiltration analgesia in rats. J Pharm Pharmacol. 73:206–11. DOI: 10.1093/jpp/rgaa021. PMID: 33793809.
Article54. Sánchez-Chapula JA, Salinas-Stefanon E, Torres-Jácome J, Benavides-Haro DE, Navarro-Polanco RA. 2001; Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J Pharmacol Exp Ther. 297:437–45. PMID: 11259572.55. Lee W, Ruijgrok L, Boxma-de Klerk B, Kok MR, Kloppenburg M, Gerards A, et al. 2018; Efficacy of hydroxychloroquine in hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken). 70:1320–5. DOI: 10.1002/acr.23471. PMID: 29125901.
Article56. Kingsbury SR, Tharmanathan P, Keding A, Ronaldson SJ, Grainger A, Wakefield RJ, et al. 2018; Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis: a randomized trial. Ann Intern Med. 168:385–95. DOI: 10.7326/M17-1430. PMID: 29459986.
Article57. Ronaldson SJ, Keding A, Tharmanathan P, Arundel C, Kingsbury SR, Conaghan PG, et al. 2021; Cost-effectiveness of hydroxychloroquine versus placebo for hand osteoarthritis: economic evaluation of the HERO trial. F1000Res. 10:821. DOI: 10.12688/f1000research.55296.1. PMID: 34950454. PMCID: PMC8666991.
Article58. Williams HJ, Egger MJ, Singer JZ, Willkens RF, Kalunian KC, Clegg DO, et al. 1994; Comparison of hydroxychloroquine and placebo in the treatment of the arthropathy of mild systemic lupus erythematosus. J Rheumatol. 21:1457–62. PMID: 7983646.59. Kedor C, Detert J, Rau R, Wassenberg S, Listing J, Klaus P, et al. 2021; Hydroxychloroquine in patients with inflammatory and erosive osteoarthritis of the hands: results of the OA-TREAT study-a randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial. RMD Open. 7:e001660. DOI: 10.1136/rmdopen-2021-001660. PMID: 34215704. PMCID: PMC8256837. PMID: 2d97159671014276959a240b70ed3576.
Article60. Martí-Carvajal A, Ramon-Pardo P, Javelle E, Simon F, Aldighieri S, Horvath H, et al. 2017; Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 12:e0179028. DOI: 10.1371/journal.pone.0179028. PMID: 28609439. PMCID: PMC5469465. PMID: 66f3c39ff8d9452db920eb764546cb8e.
Article61. Rodrigo C, Herath T, Wickramarachchi U, Fernando D, Rajapakse S. 2022; Treatment of chikungunya-associated joint pain: a systematic review of controlled clinical trials. Trans R Soc Trop Med Hyg. 116:889–99. DOI: 10.1093/trstmh/trac045. PMID: 35666998.
Article62. Eisen D. 1993; Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 28:609–12. DOI: 10.1016/0190-9622(93)70082-5. PMID: 8463463.
Article63. Yeshurun A, Bergman R, Bathish N, Khamaysi Z. 2019; Hydroxychloroquine sulphate therapy of erosive oral lichen planus. Australas J Dermatol. 60:e109–12. DOI: 10.1111/ajd.12948. PMID: 30411331.
Article64. Vermeer HAB, Rashid H, Esajas MD, Oldhoff JM, Horváth B. 2021; The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 185:201–3. DOI: 10.1111/bjd.19870. PMID: 33548058. PMCID: PMC8360049.
Article65. Haight ES, Johnson EM, Carroll IR, Tawfik VL. 2020; Of mice, microglia, and (wo)men: a case series and mechanistic investigation of hydroxychloroquine for complex regional pain syndrome. Pain Rep. 5:e841. DOI: 10.1097/PR9.0000000000000841. PMID: 33490839. PMCID: PMC7808678. PMID: ed8454a3c3f045f58bf6a1e1ac2d8feb.
Article66. Li J, Kim SG, Blenis J. 2014; Rapamycin: one drug, many effects. Cell Metab. 19:373–9. DOI: 10.1016/j.cmet.2014.01.001. PMID: 24508508. PMCID: PMC3972801.
Article67. Laplante M, Sabatini DM. 2012; mTOR signaling in growth control and disease. Cell. 149:274–93. DOI: 10.1016/j.cell.2012.03.017. PMID: 22500797. PMCID: PMC3331679.
Article68. Benjamin D, Colombi M, Moroni C, Hall MN. 2011; Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 10:868–80. DOI: 10.1038/nrd3531. PMID: 22037041.
Article69. Gibbons JJ, Abraham RT, Yu K. 2009; Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 36 Suppl 3:S3–17. DOI: 10.1053/j.seminoncol.2009.10.011. PMID: 19963098.
Article70. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. RAD001 in Advanced Neuroendocrine Tumors. Third Trial (RADIANT-3) Study Group. 2011; Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 364:514–23. DOI: 10.1056/NEJMoa1009290. PMID: 21306238. PMCID: PMC4208619.
Article71. Yangyun W, Guowei S, Shufen S, Jie Y, Rui Y, Yu R. 2022; Everolimus accelerates Erastin and RSL3-induced ferroptosis in renal cell carcinoma. Gene. 809:145992. DOI: 10.1016/j.gene.2021.145992. PMID: 34648917.
Article72. Khan NA, Nikkanen J, Yatsuga S, Jackson C, Wang L, Pradhan S, et al. 2017; mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metab. 26:419–28.e5. DOI: 10.1016/j.cmet.2017.07.007. PMID: 28768179.
Article73. Kang J, Feng D, Yang F, Tian X, Han W, Jia H. 2020; Comparison of rapamycin and methylprednisolone for treating inflammatory muscle disease in a murine model of experimental autoimmune myositis. Exp Ther Med. 20:219–26. DOI: 10.3892/etm.2020.8716. PMID: 32536994. PMCID: PMC7291653.
Article74. Lilleker JB, Bukhari M, Chinoy H. 2019; Rapamycin for inclusion body myositis: targeting non-inflammatory mechanisms. Rheumatology (Oxford). 58:375–6. DOI: 10.1093/rheumatology/key043. PMID: 29529264.
Article75. Khaibullina A, Almeida LE, Wang L, Kamimura S, Wong EC, Nouraie M, et al. 2015; Rapamycin increases fetal hemoglobin and ameliorates the nociception phenotype in sickle cell mice. Blood Cells Mol Dis. 55:363–72. DOI: 10.1016/j.bcmd.2015.08.001. PMID: 26460261.
Article76. Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, et al. 2013; Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 19:603–7. DOI: 10.1038/nm.3127. PMID: 23542787.
Article77. Waldner M, Fantus D, Solari M, Thomson AW. 2016; New perspectives on mTOR inhibitors (rapamycin, rapalogs and TORKinibs) in transplantation. Br J Clin Pharmacol. 82:1158–70. DOI: 10.1111/bcp.12893. PMID: 26810941. PMCID: PMC5061789.
Article78. Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. 2012; Rapamycin slows aging in mice. Aging Cell. 11:675–82. DOI: 10.1111/j.1474-9726.2012.00832.x. PMID: 22587563. PMCID: PMC3434687.
Article79. Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA, Velarde MC, Syed FA, et al. 2019; A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun. 10:3194. DOI: 10.1038/s41467-019-11174-0. PMID: 31324799. PMCID: PMC6642166. PMID: 62fe1b728b0148059e306c8857df6c69.
Article80. Lv J, Li Z, She S, Xu L, Ying Y. 2015; Effects of intrathecal injection of rapamycin on pain threshold and spinal cord glial activation in rats with neuropathic pain. Neurol Res. 37:739–43. DOI: 10.1179/1743132815Y.0000000052. PMID: 26004146.
Article81. Feng T, Yin Q, Weng ZL, Zhang JC, Wang KF, Yuan SY, et al. 2014; Rapamycin ameliorates neuropathic pain by activating autophagy and inhibiting interleukin-1β in the rat spinal cord. J Huazhong Univ Sci Technolog Med Sci. 34:830–7. DOI: 10.1007/s11596-014-1361-6. PMID: 25480578.
Article82. Tateda S, Kanno H, Ozawa H, Sekiguchi A, Yahata K, Yamaya S, et al. 2017; Rapamycin suppresses microglial activation and reduces the development of neuropathic pain after spinal cord injury. J Orthop Res. 35:93–103. DOI: 10.1002/jor.23328. PMID: 27279283.
Article83. Zhang X, Jiang N, Li J, Zhang D, Lv X. 2019; Rapamycin alleviates proinflammatory cytokines and nociceptive behavior induced by chemotherapeutic paclitaxel. Neurol Res. 41:52–9. DOI: 10.1080/01616412.2018.1531199. PMID: 30325723.
Article84. Kwon M, Han J, Kim UJ, Cha M, Um SW, Bai SJ, et al. 2017; Inhibition of mammalian target of rapamycin (mTOR) signaling in the insular cortex alleviates neuropathic pain after peripheral nerve injury. Front Mol Neurosci. 10:79. DOI: 10.3389/fnmol.2017.00079. PMID: 28377693. PMCID: PMC5359287.
Article85. Asante CO, Wallace VC, Dickenson AH. 2010; Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat. J Pain. 11:1356–67. DOI: 10.1016/j.jpain.2010.03.013. PMID: 20452291. PMCID: PMC3000494.
Article86. Géranton SM, Jiménez-Díaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, et al. 2009; A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci. 29:15017–27. DOI: 10.1523/JNEUROSCI.3451-09.2009. PMID: 19940197. PMCID: PMC2830115.
Article87. Chen WH, Chang YT, Chen YC, Cheng SJ, Chen CC. 2018; Spinal protein kinase C/extracellular signal-regulated kinase signal pathway mediates hyperalgesia priming. Pain. 159:907–18. DOI: 10.1097/j.pain.0000000000001162. PMID: 29672451.
Article88. Lyu D, Yu W, Tang N, Wang R, Zhao Z, Xie F, et al. 2013; The mTOR signaling pathway regulates pain-related synaptic plasticity in rat entorhinal-hippocampal pathways. Mol Pain. 9:64. DOI: 10.1186/1744-8069-9-64. PMID: 24313960. PMCID: PMC3892125.
Article89. Abdelaziz DM, Stone LS, Komarova SV. 2014; Osteolysis and pain due to experimental bone metastases are improved by treatment with rapamycin. Breast Cancer Res Treat. 143:227–37. DOI: 10.1007/s10549-013-2799-0. PMID: 24327332.
Article90. Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, et al. 2014; Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest. 124:592–603. DOI: 10.1172/JCI70236. PMID: 24382350. PMCID: PMC3904613.
Article91. Lutz BM, Nia S, Xiong M, Tao YX, Bekker A. 2015; mTOR, a new potential target for chronic pain and opioid-induced tolerance and hyperalgesia. Mol Pain. 11:32. DOI: 10.1186/s12990-015-0030-5. PMID: 26024835. PMCID: PMC4455918.
Article92. Zhang J, Wang Y, Qi X. 2019; Systemic rapamycin attenuates morphine-induced analgesic tolerance and hyperalgesia in mice. Neurochem Res. 44:465–71. DOI: 10.1007/s11064-018-2699-0. PMID: 30547365.
Article93. Shirooie S, Sahebgharani M, Esmaeili J, Dehpour AR. 2019; In vitro evaluation of effects of metformin on morphine and methadone tolerance through mammalian target of rapamycin signaling pathway. J Cell Physiol. 234:3058–66. DOI: 10.1002/jcp.27125. PMID: 30146703.
Article94. Nguyen LS, Vautier M, Allenbach Y, Zahr N, Benveniste O, Funck-Brentano C, et al. 2019; Sirolimus and mTOR inhibitors: a review of side effects and specific management in solid organ transplantation. Drug Saf. 42:813–25. DOI: 10.1007/s40264-019-00810-9. PMID: 30868436.
Article95. Zhou YQ, Liu DQ, Chen SP, Sun J, Wang XM, Tian YK, et al. 2018; Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol Res. 134:305–10. DOI: 10.1016/j.phrs.2018.07.002. PMID: 30042091.
Article96. Bastos LF, Merlo LA, Rocha LT, Coelho MM. 2007; Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur J Pharmacol. 576:171–9. DOI: 10.1016/j.ejphar.2007.07.049. PMID: 17719028.
Article97. Bastos LF, Angusti A, Vilaça MC, Merlo LA, Nascimento EB Jr, Rocha LT, et al. 2008; A novel non-antibacterial, non-chelating hydroxypyrazoline derivative of minocycline inhibits nociception and oedema in mice. Br J Pharmacol. 155:714–21. DOI: 10.1038/bjp.2008.303. PMID: 18660827. PMCID: PMC2584916.
Article98. Guasti L, Richardson D, Jhaveri M, Eldeeb K, Barrett D, Elphick MR, et al. 2009; Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol Pain. 5:35. DOI: 10.1186/1744-8069-5-35. PMID: 19570201. PMCID: PMC2719614. PMID: 65c67abd410c43ea906c500ab1e1b045.
Article99. Li K, Fu KY, Light AR, Mao J. 2010; Systemic minocycline differentially influences changes in spinal microglial markers following formalin-induced nociception. J Neuroimmunol. 221:25–31. DOI: 10.1016/j.jneuroim.2010.02.003. PMID: 20202692. PMCID: PMC2874948.
Article100. Tabassum S, Misrani A, Huo Q, Ahmed A, Long C, Yang L. 2022; Minocycline ameliorates chronic unpredictable mild stress-induced neuroinflammation and abnormal mPFC-HIPP oscillations in mice. Mol Neurobiol. 59:6874–95. DOI: 10.1007/s12035-022-03018-8. PMID: 36048340.
Article101. Padi SS, Kulkarni SK. 2008; Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol. 601:79–87. DOI: 10.1016/j.ejphar.2008.10.018. PMID: 18952075.
Article102. Mika J, Rojewska E, Makuch W, Przewlocka B. 2010; Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience. 165:1420–8. DOI: 10.1016/j.neuroscience.2009.11.064. PMID: 19961904.
Article103. Yoon SY, Patel D, Dougherty PM. 2012; Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience. 221:214–24. DOI: 10.1016/j.neuroscience.2012.06.024. PMID: 22742905. PMCID: PMC3424316.
Article104. Sung CS, Cherng CH, Wen ZH, Chang WK, Huang SY, Lin SL, et al. 2012; Minocycline and fluorocitrate suppress spinal nociceptive signaling in intrathecal IL-1β-induced thermal hyperalgesic rats. Glia. 60:2004–17. DOI: 10.1002/glia.22415. PMID: 22972308.
Article105. Mei XP, Sakuma Y, Xie C, Wu D, Ho I, Kotani J, et al. 2014; Depressing interleukin-1β contributed to the synergistic effects of tramadol and minocycline on spinal nerve ligation-induced neuropathic pain. Neurosignals. 22:30–42. DOI: 10.1159/000355071. PMID: 24157594. PMID: 6e88a4f509b34c288b42bc8ef2699ec4.
Article106. Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, et al. 2010; Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol. 160:1754–64. DOI: 10.1111/j.1476-5381.2010.00811.x. PMID: 20649577. PMCID: PMC2936846.107. Ismail CAN, Ghazali AK, Suppian R, Abd Aziz CB, Long I. 2021; Minocycline alleviates nociceptive response through modulating the expression of NR2B subunit of NMDA receptor in spinal cord of rat model of painful diabetic neuropathy. J Diabetes Metab Disord. 20:793–803. DOI: 10.1007/s40200-021-00820-4. PMID: 34178864. PMCID: PMC8212342.
Article108. Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. 2010; Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 68:360–8. DOI: 10.1002/ana.22082. PMID: 20818791. PMCID: PMC4836445.109. Wang HL, Liu H, Xue ZG, Liao QW, Fang H. 2016; Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med. 20:1632–9. DOI: 10.1111/jcmm.12854. PMID: 27061744. PMCID: PMC4988280.
Article110. Takazawa T, Horiuchi T, Orihara M, Nagumo K, Tomioka A, Ideno Y, et al. 2023; Prevention of postoperative cognitive dysfunction by minocycline in elderly patients after total knee arthroplasty: a randomized, double-blind, placebo-controlled clinical trial. Anesthesiology. 138:172–83. DOI: 10.1097/ALN.0000000000004439. PMID: 36538374.
Article111. Lin CS, Tsaur ML, Chen CC, Wang TY, Lin CF, Lai YL, et al. 2007; Chronic intrathecal infusion of minocycline prevents the development of spinal-nerve ligation-induced pain in rats. Reg Anesth Pain Med. 32:209–16. DOI: 10.1016/j.rapm.2007.01.005. PMID: 17543815.
Article112. Taguchi T, Katanosaka K, Yasui M, Hayashi K, Yamashita M, Wakatsuki K, et al. 2015; Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain. 156:415–27. DOI: 10.1097/01.j.pain.0000460334.49525.5e. PMID: 25599239.
Article113. Cata JP, Weng HR, Dougherty PM. 2008; The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res. 1229:100–10. DOI: 10.1016/j.brainres.2008.07.001. PMID: 18652810. PMCID: PMC2577234.
Article114. Masocha W. 2014; Paclitaxel-induced hyposensitivity to nociceptive chemical stimulation in mice can be prevented by treatment with minocycline. Sci Rep. 4:6719. DOI: 10.1038/srep06719. PMID: 25335491. PMCID: PMC4205835.
Article115. Ismail CAN, Suppian R, Aziz CBA, Long I. 2019; Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J Diabetes Metab Disord. 18:181–90. DOI: 10.1007/s40200-019-00411-4. PMID: 31275889. PMCID: PMC6582076.
Article116. Amorim D, Puga S, Bragança R, Braga A, Pertovaara A, Almeida A, et al. 2017; Minocycline reduces mechanical allodynia and depressive-like behaviour in type-1 diabetes mellitus in the rat. Behav Brain Res. 327:1–10. DOI: 10.1016/j.bbr.2017.03.003. PMID: 28286285.
Article117. Miranda HF, Sierralta F, Jorquera V, Poblete P, Prieto JC, Noriega V. 2017; Antinociceptive interaction of gabapentin with minocycline in murine diabetic neuropathy. Inflammopharmacology. 25:91–7. Erratum in: Inflammopharmacology 2017; 25: 485. DOI: 10.1007/s10787-017-0308-5. PMID: 28155118.
Article118. Bastos LF, Prazeres JD, Godin AM, Menezes RR, Soares DG, Ferreira WC, et al. 2013; Sex-independent suppression of experimental inflammatory pain by minocycline in two mouse strains. Neurosci Lett. 553:110–4. DOI: 10.1016/j.neulet.2013.08.026. PMID: 23973305.
Article119. Cho IH, Chung YM, Park CK, Park SH, Lee H, Kim D, et al. 2006; Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat. Brain Res. 1072:208–14. Erratum in: Brain Res 2012; 1464: 89. DOI: 10.1016/j.brainres.2012.05.003. PMID: 16427032.
Article120. Cho IH, Lee MJ, Jang M, Gwak NG, Lee KY, Jung HS. 2012; Minocycline markedly reduces acute visceral nociception via inhibiting neuronal ERK phosphorylation. Mol Pain. 8:13. DOI: 10.1186/1744-8069-8-13. PMID: 22364340. PMCID: PMC3342906. PMID: c986648b7f3445399c0efb8304f50216.
Article121. Kannampalli P, Pochiraju S, Bruckert M, Shaker R, Banerjee B, Sengupta JN. 2014; Analgesic effect of minocycline in rat model of inflammation-induced visceral pain. Eur J Pharmacol. 727:87–98. DOI: 10.1016/j.ejphar.2014.01.026. PMID: 24485889. PMCID: PMC3984928.
Article122. Zhang G, Zhao BX, Hua R, Kang J, Shao BM, Carbonaro TM, et al. 2016; Hippocampal microglial activation and glucocorticoid receptor down-regulation precipitate visceral hypersensitivity induced by colorectal distension in rats. Neuropharmacology. 102:295–303. DOI: 10.1016/j.neuropharm.2015.11.028. PMID: 26656865.
Article123. Abu-Ghefreh AA, Masocha W. 2010; Enhancement of antinociception by coadministration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naïve mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskelet Disord. 11:276. DOI: 10.1186/1471-2474-11-276. PMID: 21122103. PMCID: PMC3009629. PMID: 0eaf074ee3de4e28842786b628110246.124. Song ZP, Xiong BR, Guan XH, Cao F, Manyande A, Zhou YQ, et al. 2016; Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes. Acta Pharmacol Sin. 37:753–62. DOI: 10.1038/aps.2016.1. PMID: 27157092. PMCID: PMC4954763.
Article125. Bu H, Shu B, Gao F, Liu C, Guan X, Ke C, et al. 2014; Spinal IFN-γ-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat. 143:255–63. DOI: 10.1007/s10549-013-2807-4. PMID: 24337539.
Article126. Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. 2014; Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun. 42:147–56. DOI: 10.1016/j.bbi.2014.06.015. PMID: 24994592.
Article127. Gajbhiye S, Bhangre A, Tripathi RK, Jalgaonkar S, Shankar A, Koli PG. 2022; Evaluation of antidepressant effect of minocycline in alcohol abstinence-induced depression model in mice. Cureus. 14:e28711. DOI: 10.7759/cureus.28711. PMID: 36211101. PMCID: PMC9529019.
Article128. Sumitani M, Ueda H, Hozumi J, Inoue R, Kogure T, Yamada Y, et al. 2016; Minocycline does not decrease intensity of neuropathic pain intensity, but does improve its affective dimension. J Pain Palliat Care Pharmacother. 30:31–5. DOI: 10.3109/15360288.2014.1003674. PMID: 25700217.129. Habibi-Asl B, Hassanzadeh K, Charkhpour M. 2009; Central administration of minocycline and riluzole prevents morphine-induced tolerance in rats. Anesth Analg. 109:936–42. DOI: 10.1213/ane.0b013e3181ae5f13. PMID: 19690270.
Article130. Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B. 2009; Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun. 23:75–84. DOI: 10.1016/j.bbi.2008.07.005. PMID: 18684397.
Article131. Shin DA, Kim TU, Chang MC. 2021; Minocycline for controlling neuropathic pain: a systematic narrative review of studies in humans. J Pain Res. 14:139–45. DOI: 10.2147/JPR.S292824. PMID: 33536779. PMCID: PMC7849188.
Article132. Pachman DR, Dockter T, Zekan PJ, Fruth B, Ruddy KJ, Ta LE, et al. 2017; A pilot study of minocycline for the prevention of paclitaxel-associated neuropathy:. ACCRU study RU221408I. Support Care Cancer. 25:3407–16. DOI: 10.1007/s00520-017-3760-2. PMID: 28551844.133. Wang XS, Shi Q, Bhadkamkar NA, Cleeland CS, Garcia-Gonzalez A, Aguilar JR, et al. 2019; Minocycline for symptom reduction during oxaliplatin-based chemotherapy for colorectal cancer: a phase II randomized clinical trial. J Pain Symptom Manage. 58:662–71. DOI: 10.1016/j.jpainsymman.2019.06.018. PMID: 31254639. PMCID: PMC6754803.
Article134. Wang XS, Shi Q, Mendoza T, Lin S, Chang JY, Bokhari RH, et al. 2020; Minocycline reduces chemoradiation-related symptom burden in patients with non-small cell lung cancer: a phase 2 randomized trial. Int J Radiat Oncol Biol Phys. 106:100–7. DOI: 10.1016/j.ijrobp.2019.10.010. PMID: 31627177. PMCID: PMC7043289.
Article135. Martinez V, Szekely B, Lemarié J, Martin F, Gentili M, Ben Ammar S, et al. 2013; The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. Pain. 154:1197–203. DOI: 10.1016/j.pain.2013.03.028. PMID: 23706627.
Article136. Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, et al. 2015; Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology. 122:399–406. DOI: 10.1097/ALN.0000000000000508. PMID: 25373391.137. Syngle A, Verma I, Krishan P, Garg N, Syngle V. 2014; Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol Sci. 35:1067–73. DOI: 10.1007/s10072-014-1647-2. PMID: 24497205.
Article138. Narang T, Dogra S. Arshdeep. 2017; Minocycline in leprosy patients with recent onset clinical nerve function impairment. Dermatol Ther. doi: 10.1111/dth.12404. DOI: 10.1111/dth.12404. PMID: 27550711.
Article139. Curtin CM, Kenney D, Suarez P, Hentz VR, Hernandez-Boussard T, Mackey S, et al. 2017; A double-blind placebo randomized controlled trial of minocycline to reduce pain after carpal tunnel and trigger finger release. J Hand Surg Am. 42:166–74. DOI: 10.1016/j.jhsa.2016.12.011. PMID: 28259273.
Article140. Martins AM, Marto JM, Johnson JL, Graber EM. 2021; A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics (Basel). 10:757. DOI: 10.3390/antibiotics10070757. PMID: 34206485. PMCID: PMC8300648. PMID: da32ab3f5f504b2fb6262822bd816dde.
Article141. Wozel G, Blasum C. 2014; Dapsone in dermatology and beyond. Arch Dermatol Res. 306:103–24. DOI: 10.1007/s00403-013-1409-7. PMID: 24310318. PMCID: PMC3927068.
Article142. Wolf R, Matz H, Orion E, Tuzun B, Tuzun Y. 2002; Dapsone. Dermatol Online J. 8:2. DOI: 10.5070/D330M4B5KR. PMID: 12165212.
Article143. Khalilzadeh M, Shayan M, Jourian S, Rahimi M, Sheibani M, Dehpour AR. 2022; A comprehensive insight into the anti-inflammatory properties of dapsone. Naunyn Schmiedebergs Arch Pharmacol. 395:1509–23. DOI: 10.1007/s00210-022-02297-1. PMID: 36125533.
Article144. Suda T, Suzuki Y, Matsui T, Inoue T, Niide O, Yoshimaru T, et al. 2005; Dapsone suppresses human neutrophil superoxide production and elastase release in a calcium-dependent manner. Br J Dermatol. 152:887–95. DOI: 10.1111/j.1365-2133.2005.06559.x. PMID: 15888142.
Article145. Ruzicka T, Wasserman SI, Soter NA, Printz MP. 1983; Inhibition of rat mast cell arachidonic acid cyclooxygenase by dapsone. J Allergy Clin Immunol. 72:365–70. DOI: 10.1016/0091-6749(83)90501-8. PMID: 6413566.
Article146. Kanoh S, Tanabe T, Rubin BK. 2011; Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with lipopolysaccharide and resolves airway inflammation in the ferret. Chest. 140:980–90. DOI: 10.1378/chest.10-2908. PMID: 21436242.
Article147. Abe M, Shimizu A, Yokoyama Y, Takeuchi Y, Ishikawa O. 2008; A possible inhibitory action of diaminodiphenyl sulfone on tumour necrosis factor-alpha production from activated mononuclear cells on cutaneous lupus erythematosus. Clin Exp Dermatol. 33:759–63. DOI: 10.1111/j.1365-2230.2008.02864.x. PMID: 18713254.148. Rodríguez E, Méndez-Armenta M, Villeda-Hernández J, Galván-Arzate S, Barroso-Moguel R, Rodríguez F, et al. 1999; Dapsone prevents morphological lesions and lipid peroxidation induced by quinolinic acid in rat corpus striatum. Toxicology. 139:111–8. DOI: 10.1016/S0300-483X(99)00116-X. PMID: 10614692.
Article149. Santamaría A, Ordaz-Moreno J, Rubio-Osornio M, Solís-Hernández F, Ríos C. 1997; Neuroprotective effect of dapsone against quinolinate- and kainate-induced striatal neurotoxicities in rats. Pharmacol Toxicol. 81:271–5. PMID: 9444668.150. Mata-Bermudez A, Diaz-Ruiz A, Burelo M, García-Martínez BA, Jardon-Guadarrama G, Calderón-Estrella F, et al. 2021; Dapsone prevents allodynia and hyperalgesia and decreased oxidative stress after spinal cord injury in rats. Spine (Phila Pa 1976). 46:1287–94. DOI: 10.1097/BRS.0000000000004015. PMID: 34517396.
Article151. Ríos C, Orozco-Suarez S, Salgado-Ceballos H, Mendez-Armenta M, Nava-Ruiz C, Santander I, et al. 2015; Anti-apoptotic effects of dapsone after spinal cord injury in rats. Neurochem Res. 40:1243–51. DOI: 10.1007/s11064-015-1588-z. PMID: 25931161.
Article152. Diaz-Ruiz A, Salgado-Ceballos H, Montes S, Guizar-Sahagún G, Gelista-Herrera N, Mendez-Armenta M, et al. 2011; Delayed administration of dapsone protects from tissue damage and improves recovery after spinal cord injury. J Neurosci Res. 89:373–80. DOI: 10.1002/jnr.22555. PMID: 21259324.
Article153. Shayesteh S, Khalilzadeh M, Takzaree N, Dehpour AR. 2022; Dapsone improves the vincristine-induced neuropathic nociception by modulating neuroinflammation and oxidative stress. Daru. 30:303–10. DOI: 10.1007/s40199-022-00448-6. PMID: 36104653.
Article154. Swinson DR, Zlosnick J, Jackson L. 1981; Double-blind trial of dapsone against placebo in the treatment of rheumatoid arthritis. Ann Rheum Dis. 40:235–9. DOI: 10.1136/ard.40.3.235. PMID: 7018409. PMCID: PMC1000754.
Article155. Fowler PD, Shadforth MF, Crook PR, Lawton A. 1984; Report on chloroquine and dapsone in the treatment of rheumatoid arthritis: a 6-month comparative study. Ann Rheum Dis. 43:200–4. DOI: 10.1136/ard.43.2.200. PMID: 6370150. PMCID: PMC1001465.
Article156. Haar D, Sølvkjaer M, Unger B, Rasmussen KJ, Christensen L, Hansen TM. 1993; A double-blind comparative study of hydroxychloroquine and dapsone, alone and in combination, in rheumatoid arthritis. Scand J Rheumatol. 22:113–8. DOI: 10.3109/03009749309099254. PMID: 8316771.
Article157. Gusdorf L, Lipsker D. 2018; Neutrophilic urticarial dermatosis: a review. Ann Dermatol Venereol. 145:735–40. DOI: 10.1016/j.annder.2018.06.010. PMID: 30224079.
Article158. Shi H, Gudjonsson JE, Kahlenberg JM. 2020; Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 32:208–14. DOI: 10.1097/BOR.0000000000000704. PMID: 32141953. PMCID: PMC7357847.
Article159. Zampeli E, Moutsopoulos HM. 2019; Dapsone: an old drug effective for subacute cutaneous lupus erythematosus. Rheumatology (Oxford). 58:920–1. DOI: 10.1093/rheumatology/key434. PMID: 30615176.
Article160. Ujiie H, Shimizu T, Ito M, Arita K, Shimizu H. 2006; Lupus erythematosus profundus successfully treated with dapsone: review of the literature. Arch Dermatol. 142:399–401. DOI: 10.1001/archderm.142.3.399. PMID: 16549729.
Article161. de Risi-Pugliese T, Cohen Aubart F, Haroche J, Moguelet P, Grootenboer-Mignot S, Mathian A, et al. 2018; Clinical, histological, immunological presentations and outcomes of bullous systemic lupus erythematosus: 10 new cases and a literature review of 118 cases. Semin Arthritis Rheum. 48:83–9. DOI: 10.1016/j.semarthrit.2017.11.003. PMID: 29191376.
Article162. Lu Q, Long H, Chow S, Hidayat S, Danarti R, Listiawan Y, et al. 2021; Guideline for the diagnosis, treatment and long-term management of cutaneous lupus erythematosus. J Autoimmun. 123:102707. DOI: 10.1016/j.jaut.2021.102707. PMID: 34364171.
Article163. Diaz-Ruiz A, Nader-Kawachi J, Calderón-Estrella F, Mata-Bermudez A, Alvarez-Mejia L, Ríos C. 2022; Dapsone, more than an effective neuro and cytoprotective drug. Curr Neuropharmacol. 20:194–210. DOI: 10.2174/1570159X19666210617143108. PMID: 34139984. PMCID: PMC9199557.
Article164. Nader-Kawachi J, Góngora-Rivera F, Santos-Zambrano J, Calzada P, Ríos C. 2007; Neuroprotective effect of dapsone in patients with acute ischemic stroke: a pilot study. Neurol Res. 29:331–4. DOI: 10.1179/016164107X159234. PMID: 17509235.
Article165. Lee JH, Lee CJ, Park J, Lee SJ, Choi SH. 2021; The neuroinflammasome in Alzheimer's disease and cerebral stroke. Dement Geriatr Cogn Dis Extra. 11:159–67. DOI: 10.1159/000516074. PMID: 34249072. PMCID: PMC8255751. PMID: a5c8a32d4da94fd48eba1782c1ca6df3.
Article166. Walling HW, Sontheimer RD. 2009; Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 10:365–81. DOI: 10.2165/11310780-000000000-00000. PMID: 19824738.167. Ahrens EM, Meckler RJ, Callen JP. 1986; Dapsone-induced peripheral neuropathy. Int J Dermatol. 25:314–6. DOI: 10.1111/j.1365-4362.1986.tb02253.x. PMID: 3013789.
Article168. Gutmann L, Martin JD, Welton W. 1976; Dapsone motor neuropathy--an axonal disease. Neurology. 26(6 PT 1):514–6. DOI: 10.1212/WNL.26.6.514. PMID: 945490.
Article169. Prussick R, Shear NH. 1996; Dapsone hypersensitivity syndrome. J Am Acad Dermatol. 35(2 Pt 2):346–9. DOI: 10.1016/S0190-9622(96)90667-2. PMID: 8698924.
Article170. Zaccone G, Capillo G, Fernandes JMO, Kiron V, Lauriano ER, Alesci A, et al. 2022; Expression of the antimicrobial peptide Piscidin 1 and neuropeptides in fish gill and skin: a potential participation in neuro-immune interaction. Mar Drugs. 20:145. DOI: 10.3390/md20020145. PMID: 35200674. PMCID: PMC8879440. PMID: ba10a61b534341f7a8705c598edcd270.
Article171. Lauriano ER, Capillo G, Icardo JM, Fernandes JMO, Kiron V, Kuciel M, et al. 2021; Neuroepithelial cells (NECs) and mucous cells express a variety of neurotransmitters and neurotransmitter receptors in the gill and respiratory air-sac of the catfish Heteropneustes fossilis (Siluriformes, Heteropneustidae): a possible role in local immune defence. Zoology (Jena). 148:125958. DOI: 10.1016/j.zool.2021.125958. PMID: 34399394.
Article172. Salger SA, Cassady KR, Reading BJ, Noga EJ. 2016; A diverse family of host-defense peptides (Piscidins) exhibit specialized anti-bacterial and anti-protozoal activities in fishes. PLoS One. 11:e0159423. DOI: 10.1371/journal.pone.0159423. PMID: 27552222. PMCID: PMC4995043. PMID: 3c4c4d9f0bb84ef58a74cc542a1d6019.
Article173. Chen WF, Huang SY, Liao CY, Sung CS, Chen JY, Wen ZH. 2015; The use of the antimicrobial peptide piscidin (PCD)-1 as a novel anti-nociceptive agent. Biomaterials. 53:1–11. DOI: 10.1016/j.biomaterials.2015.02.069. PMID: 25890701.
Article174. Cheng MH, Pan CY, Chen NF, Yang SN, Hsieh S, Wen ZH, et al. 2020; Piscidin-1 induces apoptosis via mitochondrial reactive oxygen species-regulated mitochondrial dysfunction in human osteosarcoma cells. Sci Rep. 10:5045. DOI: 10.1038/s41598-020-61876-5. PMID: 32193508. PMCID: PMC7081333.
Article175. Ting CH, Chen YC, Wu CJ, Chen JY. 2016; Targeting FOSB with a cationic antimicrobial peptide, TP4, for treatment of triple-negative breast cancer. Oncotarget. 7:40329–47. DOI: 10.18632/oncotarget.9612. PMID: 27248170. PMCID: PMC5130011.176. Ban TA. 2006; The role of serendipity in drug discovery. Dialogues Clin Neurosci. 8:335–44. DOI: 10.31887/DCNS.2006.8.3/tban. PMID: 17117615. PMCID: PMC3181823.
Article177. Theuretzbacher U, Outterson K, Engel A, Karlén A. 2020; The global preclinical antibacterial pipeline. Nat Rev Microbiol. 18:275–85. DOI: 10.1038/s41579-019-0288-0. PMID: 31745331. PMCID: PMC7223541.
Article178. Mouraux A, Bannister K, Becker S, Finn DP, Pickering G, Pogatzki-Zahn E, et al. 2021; Challenges and opportunities in translational pain research - An opinion paper of the working group on translational pain research of the European pain federation (EFIC). Eur J Pain. 25:731–56. DOI: 10.1002/ejp.1730. PMID: 33625769. PMCID: PMC9290702.
Article179. Lapolla W, Digiorgio C, Haitz K, Magel G, Mendoza N, Grady J, et al. 2011; Incidence of postherpetic neuralgia after combination treatment with gabapentin and valacyclovir in patients with acute herpes zoster: open-label study. Arch Dermatol. 147:901–7. DOI: 10.1001/archdermatol.2011.81. PMID: 21482862.
Article