Cancer Res Treat.
2023 Jul;55(3):804-813. 10.4143/crt.2022.1305.
The Incidence and Risk Factors of Chronic Pulmonary Infection after Radiotherapy in Patients with Lung Cancer
- Affiliations
-
- 1Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2544162
- DOI: http://doi.org/10.4143/crt.2022.1305
Abstract
- Purpose
This study aimed to investigate cumulative incidence and risk factors associated with chronic pulmonary infection (CPI) development after radiotherapy for lung cancer.
Materials and Methods
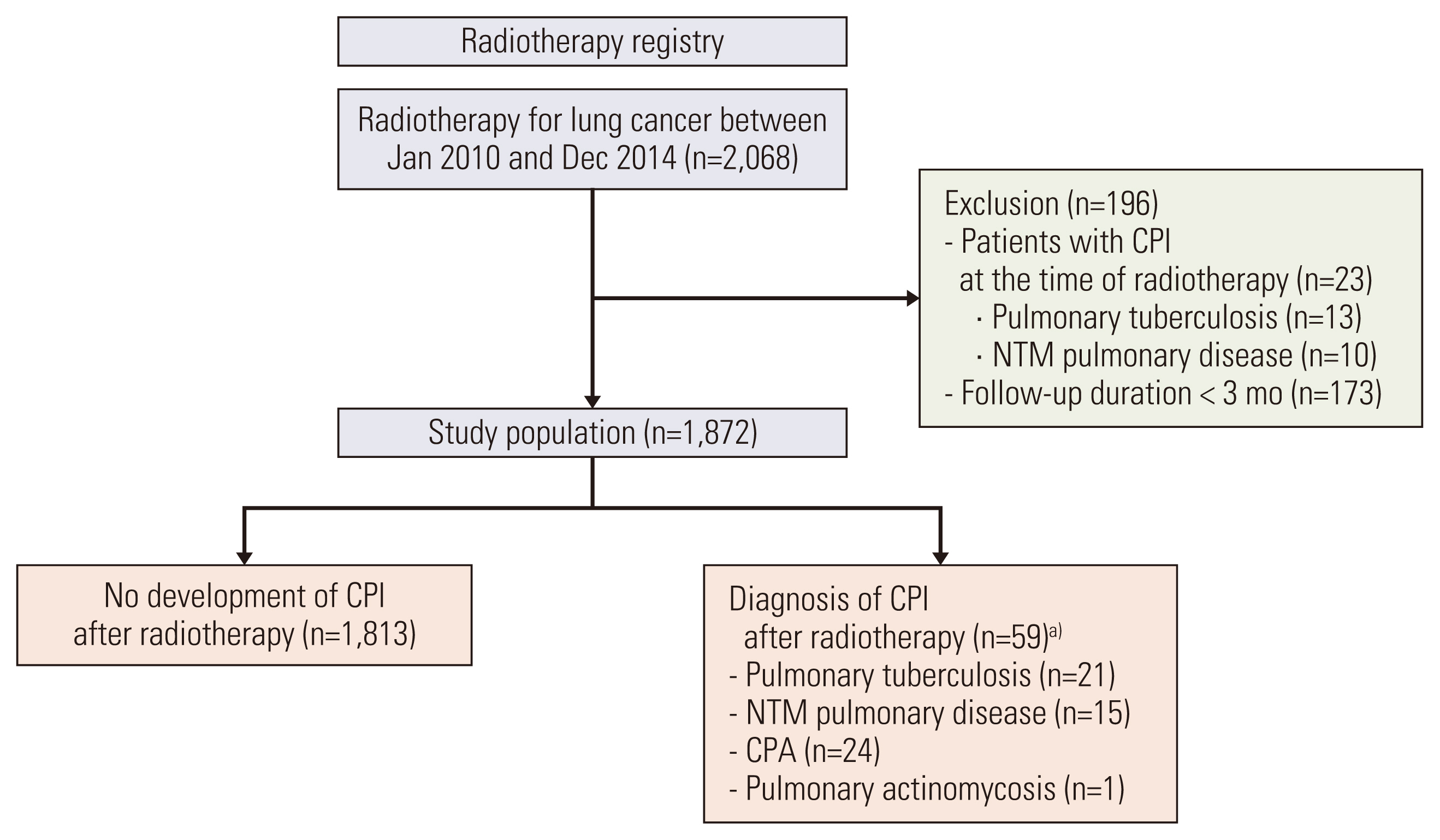
We retrospectively analyzed 1,872 patients with lung cancer who received radiotherapy for lung cancer from 2010-2014, had a follow-up period of ≥ 3 months after radiotherapy, and did not have CPI at the time of radiotherapy. CPI was defined as pulmonary tuberculosis, non-tuberculous mycobacterial pulmonary disease, chronic pulmonary aspergillosis, or pulmonary actinomycosis. The cumulative incidence of CPI and overall survival (OS) were estimated using the Kaplan-Meier method, and a multivariable Cox proportional hazards analysis was performed to identify risk factors associated with CPI development.
Results
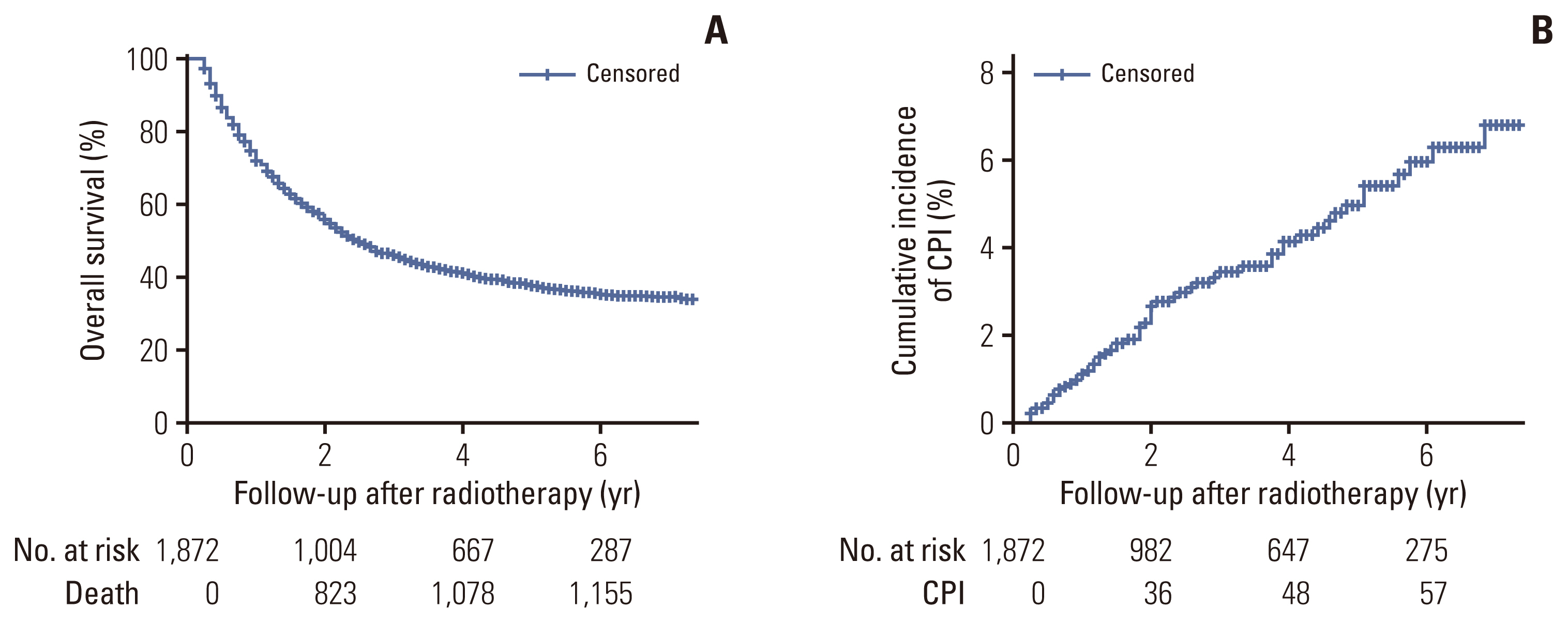
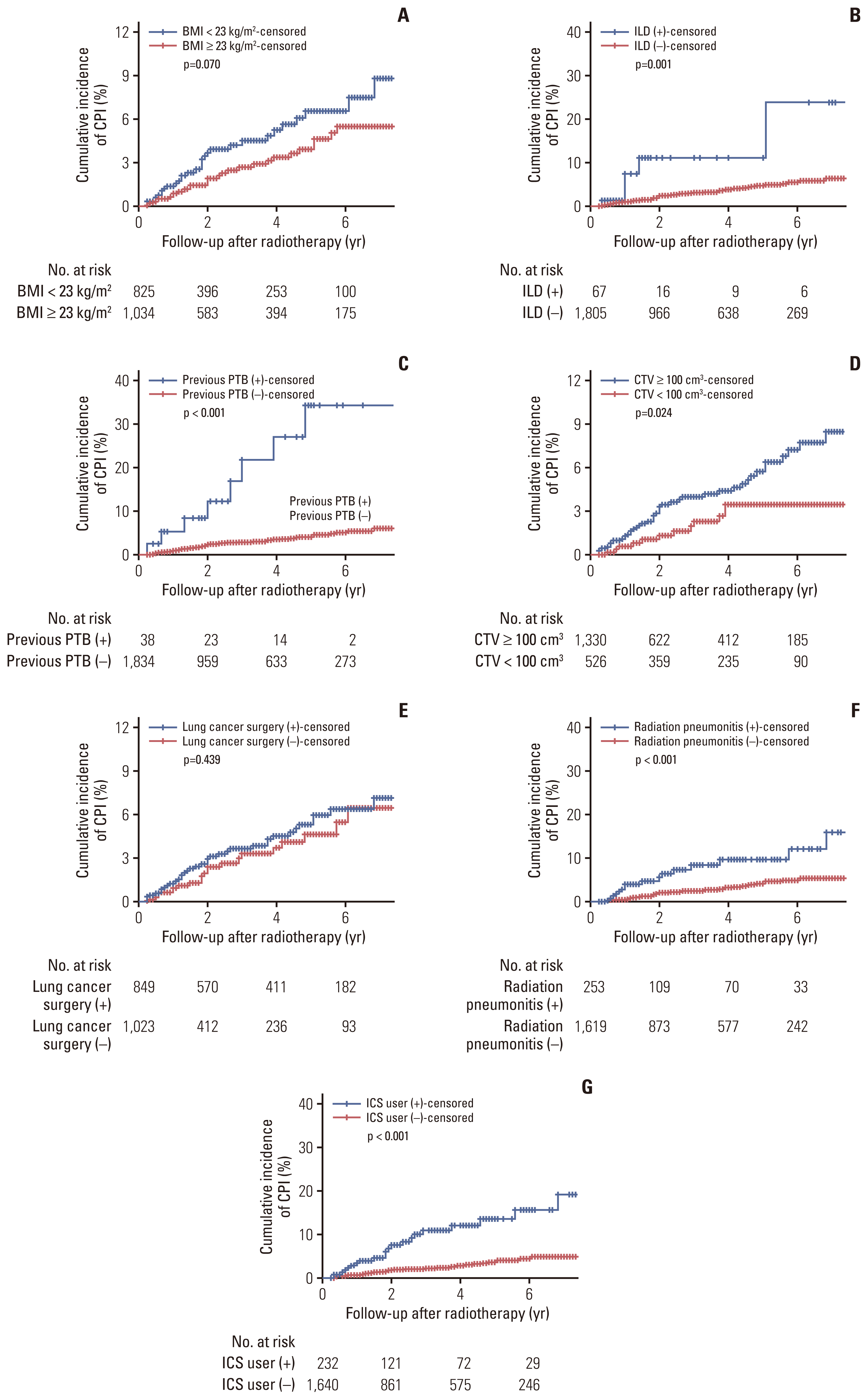
The median follow-up period was 2.3 years with OS rates of 55.6% and 37.6% at 2 and 5 years, respectively. CPI developed in 59 patients at a median of 1.8 years after radiotherapy, with cumulative incidence rates of 1.1%, 3.4%, 5.0%, and 6.8% at 1, 3, 5, and 7 years, respectively. A lower body mass index, interstitial lung disease, prior pulmonary tuberculosis, larger clinical target volume, history of lung cancer surgery or radiation pneumonitis, and use of inhaled corticosteroids were independent risk factors for CPI development.
Conclusion
The long-term survival rate of lung cancer patients receiving radiotherapy was not low, but the cumulative incidence of CPI gradually increased to 6.8% at 7 years after radiotherapy. Therefore, close monitoring of CPI development is required in surviving patients with risk factors.
Keyword
Figure
Reference
-
References
1. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020; 383:640–9.2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.3. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010; 303:1070–6.4. Donlon NE, Power R, Hayes C, Reynolds JV, Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021; 502:84–96.
Article5. Kasmann L, Dietrich A, Staab-Weijnitz CA, Manapov F, Behr J, Rimner A, et al. Radiation-induced lung toxicity: cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020; 15:214.6. Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008; 31:1322–33.
Article7. Nachiappan AC, Rahbar K, Shi X, Guy ES, Mortani Barbosa EJ Jr, Shroff GS, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics. 2017; 37:52–72.
Article8. Page ID, Byanyima R, Hosmane S, Onyachi N, Opira C, Richardson M, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019; 53:1801184.9. Dobler CC, Cheung K, Nguyen J, Martin A. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta-analysis. Eur Respir J. 2017; 50:1700157.10. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012; 185:881–6.
Article11. Tamura A, Suzuki J, Fukami T, Matsui H, Akagawa S, Ohta K, et al. Chronic pulmonary aspergillosis as a sequel to lobectomy for lung cancer. Interact Cardiovasc Thorac Surg. 2015; 21:650–6.
Article12. Shin SH, Kim BG, Kang J, Um SW, Kim H, Kim HK, et al. Incidence and risk factors of chronic pulmonary Aspergillosis development during long-term follow-up after lung cancer surgery. J Fungi (Basel). 2020; 6:271.
Article13. Definitions and reporting framework for tuberculosis—2013 revision updated December 2014 and January 2020. Geneva: World Health Organization;2020.14. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020; 56:2000535.
Article15. Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016; 47:45–68.16. Jhun BW, Jung WJ, Hwang NY, Park HY, Jeon K, Kang ES, et al. Risk factors for the development of chronic pulmonary aspergillosis in patients with nontuberculous mycobacterial lung disease. PLoS One. 2017; 12:e0188716.
Article17. Fan WC, Ting WY, Lee MC, Huang SF, Chiu CH, Lai SL, et al. Latent TB infection in newly diagnosed lung cancer patients: a multicenter prospective observational study. Lung Cancer. 2014; 85:472–8.
Article18. Evman S, Baysungur V, Alpay L, Uskul B, Misirlioglu AK, Kanbur S, et al. Management and surgical outcomes of concurrent tuberculosis and lung cancer. Thorac Cardiovasc Surg. 2017; 65:542–5.
Article19. Izumi K, Morimoto K, Hasegawa N, Uchimura K, Kawatsu L, Ato M, et al. Epidemiology of adults and children treated for nontuberculous mycobacterial pulmonary disease in Japan. Ann Am Thorac Soc. 2019; 16:341–7.
Article20. Marras TK, Vinnard C, Zhang Q, Hamilton K, Adjemian J, Eagle G, et al. Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med. 2018; 145:80–8.
Article21. Ohba H, Miwa S, Shirai M, Kanai M, Eifuku T, Suda T, et al. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med. 2012; 106:724–9.
Article22. Lai HH, Lai YJ, Yen YF. Association of body mass index with timing of death during tuberculosis treatment. PLoS One. 2017; 12:e0170104.
Article23. Jhun BW, Moon SM, Jeon K, Kwon OJ, Yoo H, Carriere KC, et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J. 2020; 55:1900798.
Article24. Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011; 37:865–72.
Article25. van Diessen JN, Chen C, van den Heuvel MM, Belderbos JS, Sonke JJ. Differential analysis of local and regional failure in locally advanced non-small cell lung cancer patients treated with concurrent chemoradiotherapy. Radiother Oncol. 2016; 118:447–52.
Article26. Koo TR, Moon SH, Lim YJ, Kim JY, Kim Y, Kim TH, et al. The effect of tumor volume and its change on survival in stage III non-small cell lung cancer treated with definitive concurrent chemoradiotherapy. Radiat Oncol. 2014; 9:283.27. Wirsdorfer F, Jendrossek V. The role of lymphocytes in radiotherapy-induced adverse late effects in the lung. Front Immunol. 2016; 7:591.28. Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013; 68:1029–36.
Article29. Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013; 68:1105–13.30. Andrejak C, Nielsen R, Thomsen VO, Duhaut P, Sorensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013; 68:256–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interstitial Lung Disease and Lung Cancer Development: A 5-Year Nationwide Population-Based Study
- Incidence and Risk Factors of Postpneumonectomy Pulmonary Edema with Non-Small Cell Lung Cancer: A Retrospective Analysis
- Obstructive Ventilatory Impairment as a Risk Factor of Lung Cancer
- The Role of Radiotherapy in Lung Cancer
- Complications of Chronic Obstructive Pulmonary Disease (COPD)