Cancer Res Treat.
2023 Jul;55(3):737-745. 10.4143/crt.2022.1414.
Identification of Genes Involved in EGF-induced Apoptosis Using CRISPR/Cas9 Knockout Screening: Implications for Novel Therapeutic Targets in EGFR-Overexpressing Cancers
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Radiation Oncology, Soonchunhyang University Seoul Hospital, Seoul, Korea
- 4Department of Radiation Oncology, Seoul National University Hospital, Seoul, Korea
- 5Department of Biomedical Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- 7Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea
- KMID: 2544157
- DOI: http://doi.org/10.4143/crt.2022.1414
Abstract
- Purpose
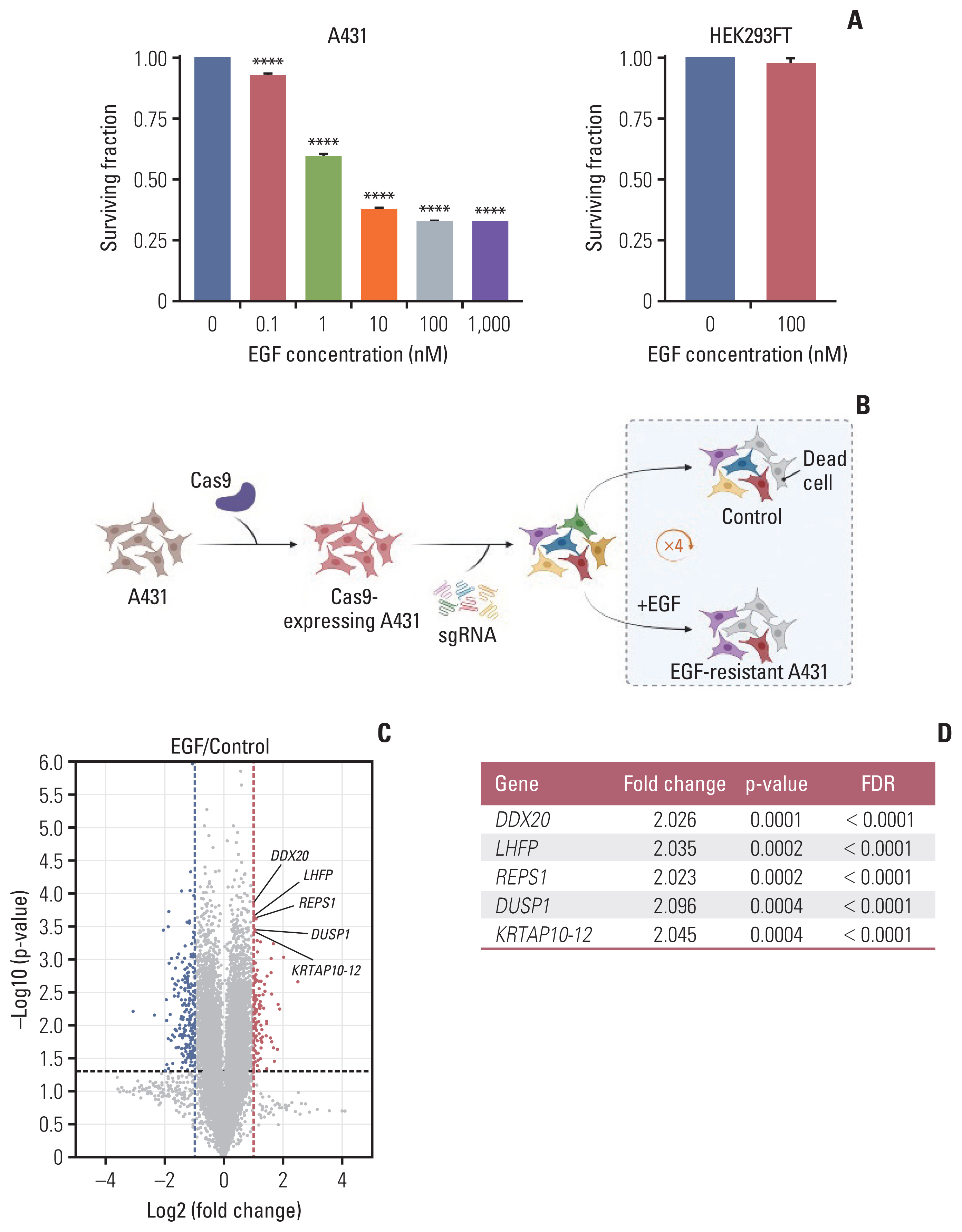
Exogenous epidermal growth factor (EGF) causes apoptosis in EGF receptor (EGFR)–overexpressing cell lines. The apoptosis-inducing factors could be a therapeutic target. We aimed to determine the mechanism of EGF-induced apoptosis using a genome-wide clustered regularly interspaced short palindromic repeats (CRISPR)-based knockout screen.
Materials and Methods
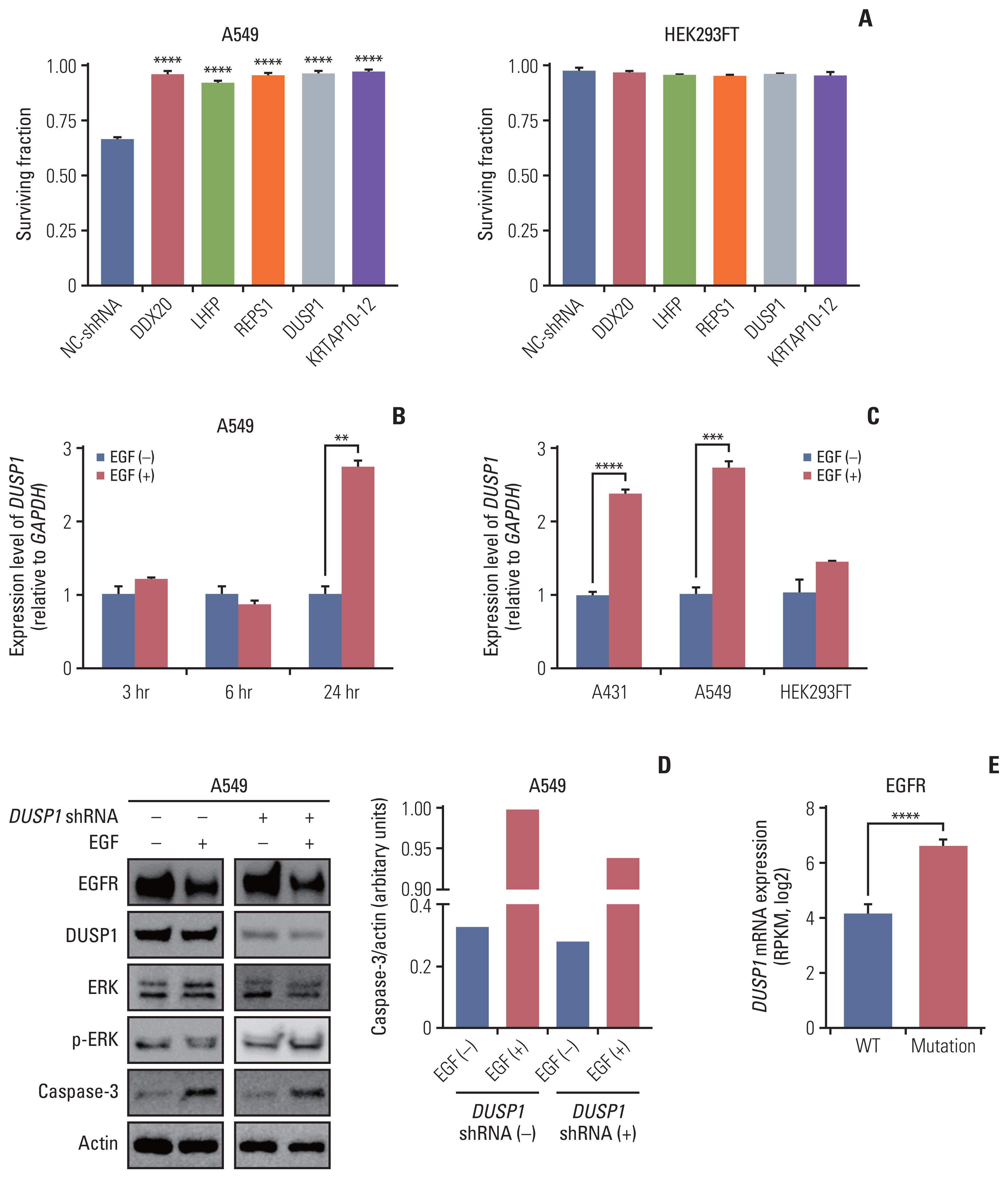
Two-vector system of the human genome-scale CRISPR knockout library v2 was used to target 19,050 genes using 123,411 single guide RNAs (sgRNAs). Recombinant human EGF (100 nM) or distilled water four times was administered to the experimental and control groups, respectively. The read counts of each sgRNA obtained from next-generation sequencing were analyzed using the edgeR algorithm. We used another EGFR-overexpressing cell line (A549) and short hairpin RNAs (shRNAs) targeting five EGF-resistance genes for validation. DUSP1 expression in A431, A549, and HEK293FT cells was calculated using reverse transcription–quantitative polymerase chain reaction.
Results
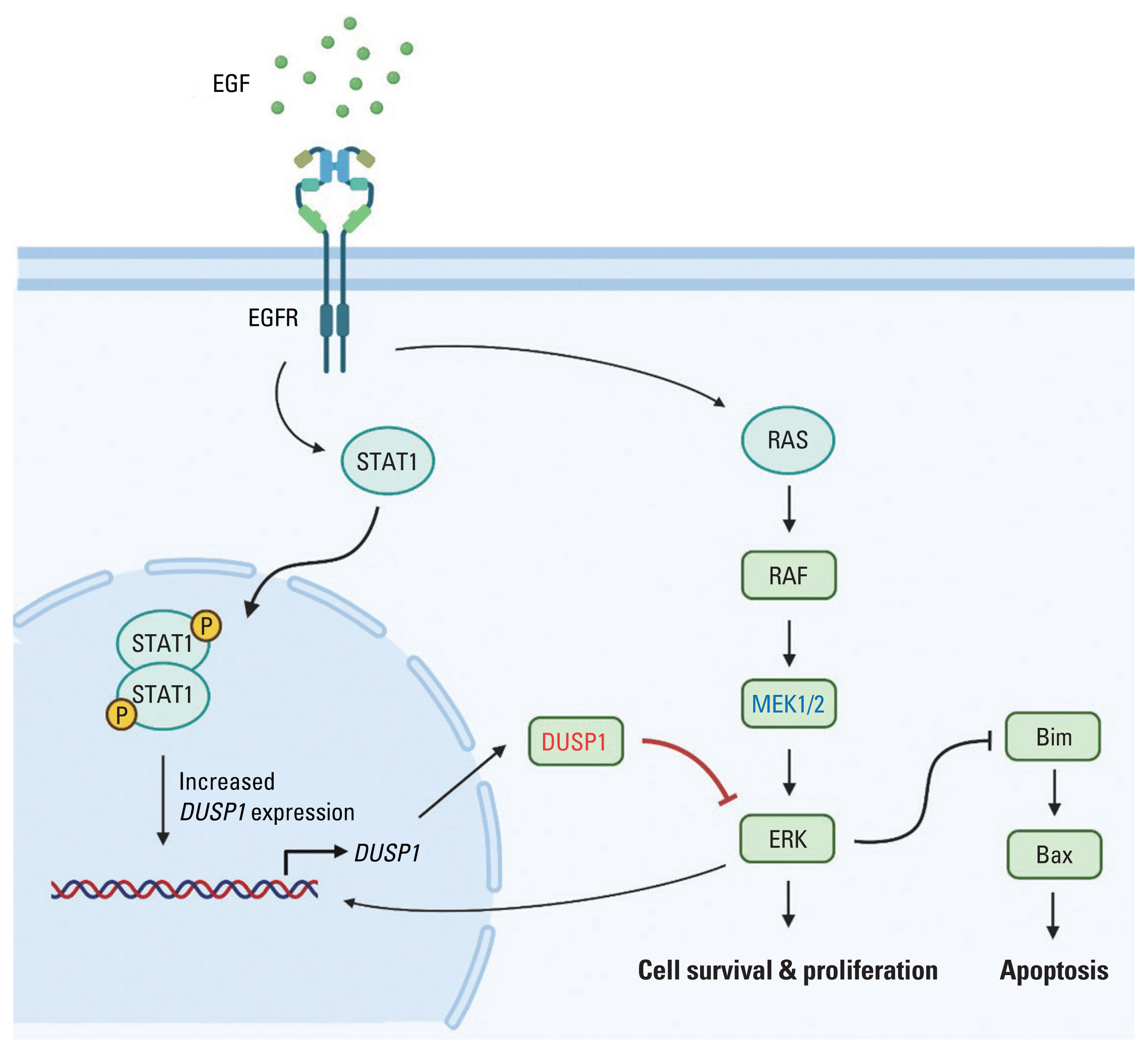
We found 77 enriched and 189 depleted genes in the experimental group using the CRISPR-based knockout screen and identified the top five EGF-resistance genes: DDX20, LHFP, REPS1, DUSP1,<.i> and KRTAP10-12. Transfecting shRNAs targeting these genes into A549 cells significantly increased the surviving fractions after EGF treatment, compared with those observed in the control shRNA-transfected cells. The expression ratio of DUSP1 (inhibits ERK signaling) increased in A431 and A549 cells after EGF treatment. However, DUSP1 expression remained unchanged in HEK293FT cells after EGF treatment.
Conclusion
The CRISPR-based knockout screen revealed 266 genes possibly responsible for EGF-induced apoptosis. DUSP1 might be a critical component of EGF-induced apoptosis and a novel target for EGFR-overexpressing cancers.
Keyword
Figure
Reference
-
References
1. Bodnar RJ. Epidermal growth factor and epidermal growth factor receptor: the yin and yang in the treatment of cutaneous wounds and cancer. Adv Wound Care (New Rochelle). 2013; 2:24–9.
Article2. Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007; 68:1110–20.
Article3. Lee S, Wu H, Song S, Kim Y, Oh Y, Lee C, et al. The therapeutic effect of recombinant human epidermal growth factor (rhEGF) on mucositis in patients with head and neck cancer undergoing radiotherapy with or without chemotherapy: a double-blind placebo-controlled prospective phase II multi-institutional clinical trial. Int J Radiat Oncol Biol Phys. 2008; 72(1 Suppl):S32.
Article4. Wu HG, Song SY, Kim YS, Oh YT, Lee CG, Keum KC, et al. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double-blind placebo-controlled prospective phase 2 multi-institutional clinical trial. Cancer. 2009; 115:3699–708.
Article5. Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005; 5:341–54.
Article6. Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010; 277:301–8.
Article7. Agarwal V, Subash A, Nayar RC, Rao V. Is EGFR really a therapeutic target in head and neck cancers? J Surg Oncol. 2019; 119:685–6.
Article8. Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003; 21:3798–807.
Article9. Thomas R, Weihua Z. Rethink of EGFR in cancer with its kinase independent function on board. Front Oncol. 2019; 9:800.
Article10. Chen Z, Chen Q, Cheng Z, Gu J, Feng W, Lei T, et al. Long non-coding RNA CASC9 promotes gefitinib resistance in NSCLC by epigenetic repression of DUSP1. Cell Death Dis. 2020; 11:858.
Article11. Gill GN, Lazar CS. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981; 293:305–7.
Article12. Choi J, Moon SY, Hong JP, Song JY, Oh KT, Lee SW. Epidermal growth factor induces cell death in the absence of overexpressed epidermal growth factor receptor and ErbB2 in various human cancer cell lines. Cancer Invest. 2010; 28:505–14.
Article13. Kim K, Wu HG, Jeon SR. Epidermal growth factor-induced cell death and radiosensitization in epidermal growth factor receptor-overexpressing cancer cell lines. Anticancer Res. 2015; 35:245–53.14. Lim YJ, Jeon SR, Koh JM, Wu HG. Tumor growth suppression and enhanced radioresponse by an exogenous epidermal growth factor in mouse xenograft models with A431 cells. Cancer Res Treat. 2015; 47:921–30.15. Grudinkin PS, Zenin VV, Kropotov AV, Dorosh VN, Nikolsky NN. EGF-induced apoptosis in A431 cells is dependent on STAT1, but not on STAT3. Eur J Cell Biol. 2007; 86:591–603.16. Kozyulina PY, Okorokova LS, Nikolsky NN, Grudinkin PS. p38 MAP kinase enhances EGF-induced apoptosis in A431 carcinoma cells by promoting tyrosine phosphorylation of STAT1. Biochem Biophys Res Commun. 2013; 430:331–5.
Article17. Alanazi I, Hoffmann P, Adelson DL. MicroRNAs are part of the regulatory network that controls EGF induced apoptosis, including elements of the JAK/STAT pathway, in A431 cells. PLoS One. 2015; 10:e0120337.
Article18. Ali R, Brown W, Purdy SC, Davisson VJ, Wendt MK. Biased signaling downstream of epidermal growth factor receptor regulates proliferative versus apoptotic response to ligand. Cell Death Dis. 2018; 9:976.
Article19. Bock C, Datlinger P, Chardon F, Coelho MA, Dong MB, Lawson KA, et al. High-content CRISPR screening. Nat Rev Methods Prim. 2022; 2:8.
Article20. Joung J, Konermann S, Gootenberg JS, Abudayyeh OO, Platt RJ, Brigham MD, et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017; 12:828–63.
Article21. Lembach KJ. Induction of human fibroblast proliferation by epidermal growth factor (EGF): enhancement by an EGF-binding arginine esterase and by ascorbate. Proc Natl Acad Sci U S A. 1976; 73:183–7.
Article22. Gil-Araujo B, Toledo Lobo MV, Gutierrez-Salmeron M, Gutierrez-Pitalua J, Ropero S, Angulo JC, et al. Dual specificity phosphatase 1 expression inversely correlates with NF-kappaB activity and expression in prostate cancer and promotes apoptosis through a p38 MAPK dependent mechanism. Mol Oncol. 2014; 8:27–38.
Article23. Seternes OM, Kidger AM, Keyse SM. Dual-specificity MAP kinase phosphatases in health and disease. Biochim Biophys Acta Mol Cell Res. 2019; 1866:124–43.
Article24. Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013; 54:1–16.
Article25. Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007; 6:391–403.
Article26. Pan W, Han J, Wei N, Wu H, Wang Y, Sun J. LINC00702-mediated DUSP1 transcription in the prevention of bladder cancer progression: Implications in cancer cell proliferation and tumor inflammatory microenvironment. Genomics. 2022; 114:110428.
Article27. Lee S, Hwang Y, Kim TH, Jeong J, Choi D, Hwang J. UPF1 inhibits hepatocellular carcinoma growth through DUSP1/p53 signal pathway. Biomedicines. 2022; 10:793.
Article28. Sanders B, McMellen A, Woodruff E, Yamamoto T, Berning A, Post M, et al. DUSP1 inhibition in the treatment of high grade serous ovarian carcinoma (141.5). Gynecol Oncol. 2022; 166(Suppl 1):S88.
Article29. Liu C, Chen Q, Liu H. ANGPTL2 aggravates doxorubicin-induced cardiotoxicity via inhibiting DUSP1 pathway. Biosci Biotechnol Biochem. 2022; 86:1631–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genome editing: the road of CRISPR/Cas9 from bench to clinic
- Construction of a CRISPR/Cas9-Mediated Genome Editing System in Lentinula edodes
- BaSDAS: a web-based pooled CRISPR-Cas9 knockout screening data analysis system
- Application of CRISPR-Cas9 gene editing for congenital heart disease
- Applications of CRISPR/Cas9 for Gene Editing in Hereditary Movement Disorders