J Korean Med Sci.
2023 May;38(20):e155. 10.3346/jkms.2023.38.e155.
Immune Responses After Vaccination With Primary 2-Dose ChAdOx1 Plus a Booster of BNT162b2 or Vaccination With Primary 2-Dose BNT162b2 Plus a Booster of BNT162b2 and the Occurrence of Omicron Breakthrough Infection
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea
- 2Department of Pediatrics, Chung-Ang University Hospital, Seoul, Korea
- 3Department of Laboratory Medicine, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Korea
- 4Division of Infectious Diseases, Department of Internal Medicine, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Korea
- 5Department of Applied Statistics, Chung-Ang University, Seoul, Korea
- KMID: 2542594
- DOI: http://doi.org/10.3346/jkms.2023.38.e155
Abstract
- Background
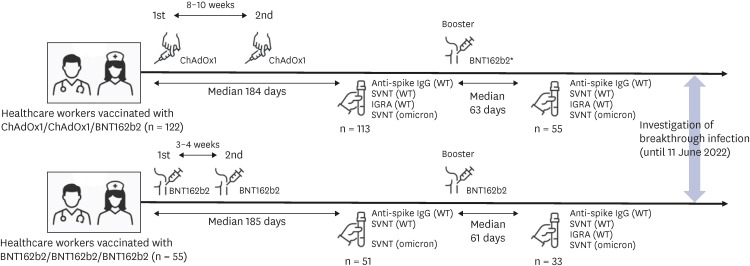
Before the omicron era, health care workers were usually vaccinated with either the primary 2-dose ChAdOx1 nCoV-19 (Oxford-AstraZeneca) series plus a booster dose of BNT162b2 (Pfizer-BioNTech) (CCB group) or the primary 2-dose BNT162b2 series plus a booster dose of BNT162b2 (BBB group) in Korea.
Methods
The two groups were compared using quantification of the surrogate virus neutralization test for wild type severe acute respiratory syndrome coronavirus 2 (SVNT-WT), the omicron variant (SVNT-O), spike-specific IgG, and interferon-gamma (IFN-γ), as well as the omicron breakthrough infection cases.
Results
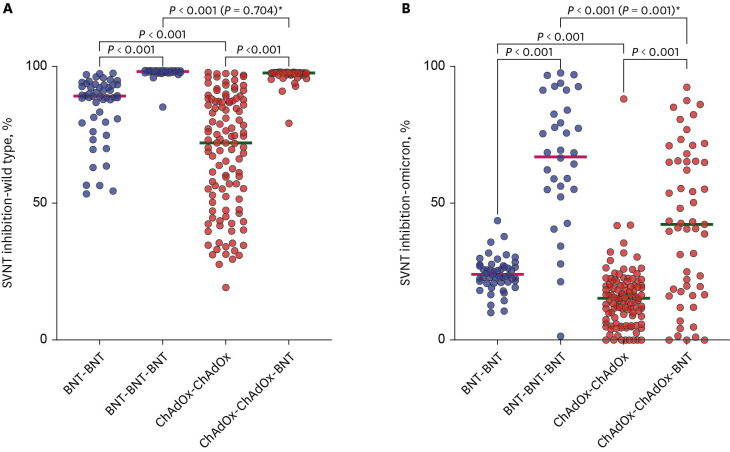
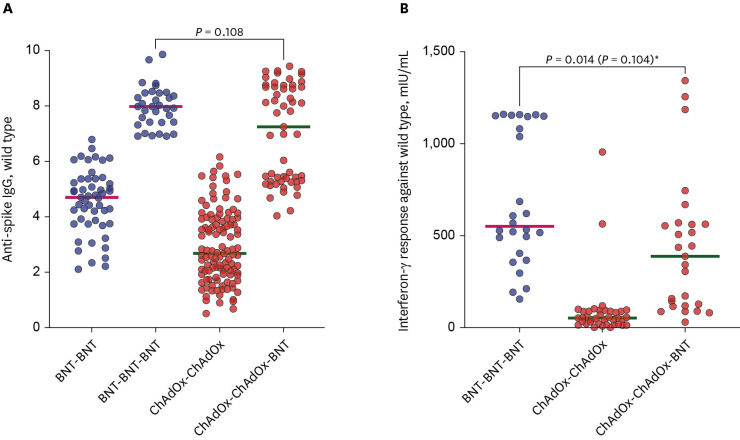
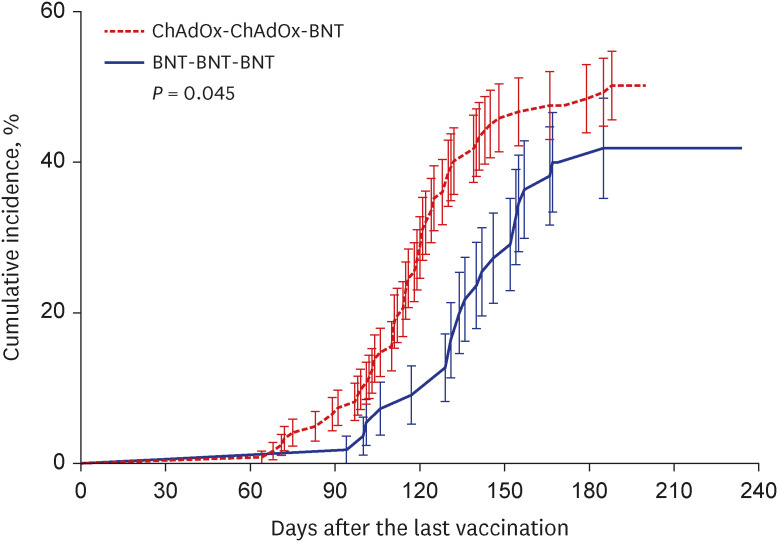
There were 113 participants enrolled in the CCB group and 51 enrolled in the BBB group. Before and after booster vaccination, the median SVNT-WT and SVNT-O values were lower in the CCB (SVNT-WT [before-after]: 72.02–97.61%, SVNT-O: 15.18–42.29%) group than in the BBB group (SVNT-WT: 89.19–98.11%, SVNT-O: 23.58–68.56%; all P < 0.001). Although the median IgG concentrations were different between the CCB and BBB groups after the primary series (2.677 vs. 4.700 AU/mL, respectively, P < 0.001), they were not different between the two groups after the booster vaccination (7.246 vs. 7.979 AU/mL, respectively, P = 0.108). In addition, the median IFN-γ concentration was higher in the BBB group than in the CCB group (550.5 and 387.5 mIU/mL, respectively, P = 0.014). There was also a difference in the cumulative incidence curves over time (CCB group 50.0% vs. BBB group 41.8%; P = 0.045), indicating that breakthrough infection occurred faster in the CCB group.
Conclusion
The cellular and humoral immune responses were low in the CCB group so that the breakthrough infection occurred faster in the CCB group than in the BBB group.
Keyword
Figure
Reference
-
1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021; 19(3):141–154. PMID: 33024307.2. Shah AS, Wood R, Gribben C, Caldwell D, Bishop J, Weir A, et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ. 2020; 371:m3582. PMID: 33115726.3. Choi SH, Park JY, Park JH, Kim MC, Kweon OJ, Chung JW. Investigation of the neutralizing antibody response of healthcare workers at a Korean university hospital six months after the introduction of the COVID-19 vaccine. Ann Lab Med. 2022; 42(5):612–615. PMID: 35470282.4. Kim YW, Kim YM, Lee SM, Lim JH, Lim DH, Park YH. Omicron dominance period (2022. 2. 1.–14.) analysis of the current status of COVID-19 confirmed cases and cohabitants and related factors. Public Health Wkly Rep. 2022; 15(15):951–955.5. EUROIMMUN. Quan-T-Cell SARS-CoV-2 & Quan-T-Cell ELISA. Updated 2021. Accessed December 11, 2022. http://www.coronavirusdiagnostics.com/documents/Indications/Infections/Coronavirus/ET_2606_D_UK_A.pdf .6. Munro AP, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021; 398(10318):2258–2276. PMID: 34863358.7. Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022; 3(1):e52–e61. PMID: 34806056.8. Qu P, Faraone J, Evans JP, Zou X, Zheng YM, Carlin C, et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022; 386(26):2526–2528. PMID: 35704428.9. Qu P, Faraone JN, Evans JP, Zheng YM, Yu L, Ma Q, et al. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022; 387(14):1329–1331. PMID: 36069925.10. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021; 27(7):1205–1211. PMID: 34002089.11. Kang YM, Minn D, Lim J, Lee KD, Jo DH, Choe KW, et al. Comparison of antibody response elicited by ChAdOx1 and BNT162b2 COVID-19 vaccine. J Korean Med Sci. 2021; 36(46):e311. PMID: 34845875.12. Hulme WJ, Williamson EJ, Green AC, Bhaskaran K, McDonald HI, Rentsch CT, et al. Comparative effectiveness of ChAdOx1 versus BNT162b2 COVID-19 vaccines in health and social care workers in England: cohort study using OpenSAFELY. BMJ. 2022; 378:e068946. PMID: 35858680.13. Xie J, Feng S, Li X, Gea-Mallorquí E, Prats-Uribe A, Prieto-Alhambra D. Comparative effectiveness of the BNT162b2 and ChAdOx1 vaccines against COVID-19 in people over 50. Nat Commun. 2022; 13(1):1519. PMID: 35314696.14. Horne EM, Hulme WJ, Keogh RH, Palmer TM, Williamson EJ, Parker EP, et al. Waning effectiveness of BNT162b2 and ChAdOx1 COVID-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records. BMJ. 2022; 378:e071249. PMID: 35858698.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the rapidity of SARS-CoV-2 immune responses between primary and booster vaccination for COVID-19
- Comparison of Antibody and T Cell Responses Induced by Single Doses of ChAdOx1 nCoV-19 and BNT162b2 Vaccines
- Changes in SARS-CoV-2 antibody titers 6 months after the booster dose of BNT162b2 COVID-19 vaccine among health care workers
- Evaluation of Cellular Responses to ChAdOx1-nCoV-19 and BNT162b2 Vaccinations
- Effects of Omicron Infection and Changes in Serum Antibody Response to Wild-Type, Delta, and Omicron After a Booster Dose With BNT163b2 Vaccine in Korean Healthcare Workers