Diabetes Metab J.
2023 May;47(3):333-344. 10.4093/dmj.2022.0348.
Gestational Diabetes Mellitus and Its Implications across the Life Span
- Affiliations
-
- 1Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada
- 2Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada
- 3Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, Toronto, ON, Canada
- 4Division of Endocrinology, University of Toronto, Toronto, ON, Canada
- 5Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, ON, Canada
- KMID: 2542510
- DOI: http://doi.org/10.4093/dmj.2022.0348
Abstract
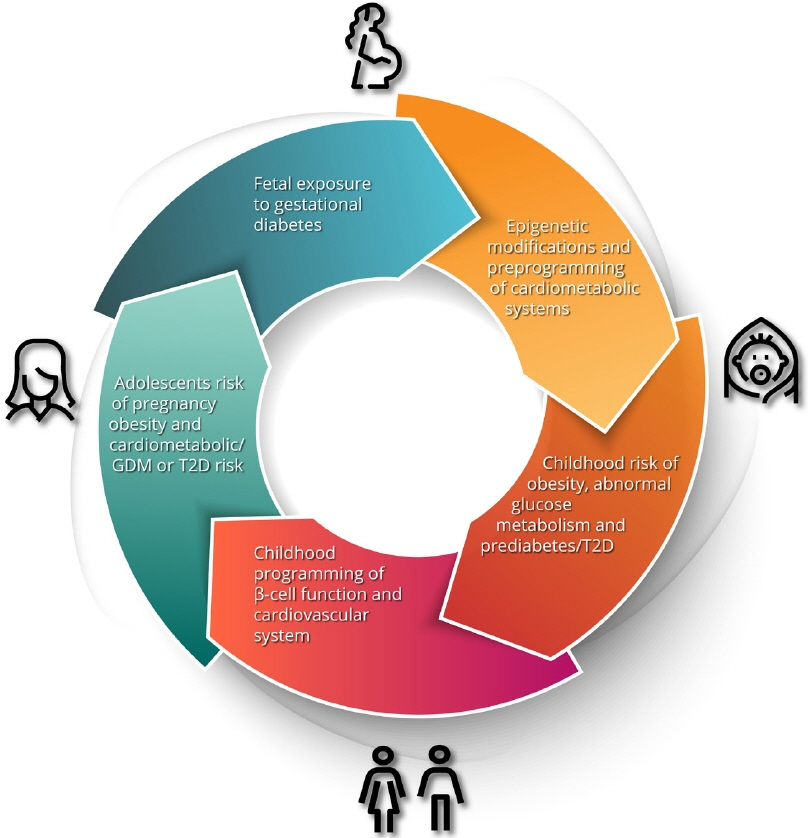
- Gestational diabetes mellitus (GDM) has historically been perceived as a medical complication of pregnancy that also serves as a harbinger of maternal risk of developing type 2 diabetes mellitus (T2DM) in the future. In recent decades, a growing body of evidence has detailed additional lifelong implications that extend beyond T2DM, including an elevated risk of ultimately developing cardiovascular disease. Furthermore, the risk factors that mediate this lifetime cardiovascular risk are evident not only after delivery but are present even before the pregnancy in which GDM is first diagnosed. The concept thus emerging from these data is that the diagnosis of GDM enables the identification of women who are already on an enhanced track of cardiometabolic risk that starts early in life. Studies of the offspring of pregnancies complicated by diabetes now suggest that the earliest underpinnings of this cardiometabolic risk profile may be determined in utero and may first manifest clinically in childhood. Accordingly, from this perspective, GDM is now seen as a chronic metabolic disorder that holds implications across the life span of both mother and child.
Figure
Cited by 1 articles
-
Amelioration of Insulin Resistance after Delivery Is Associated with Reduced Risk of Postpartum Diabetes in Women with Gestational Diabetes Mellitus
Heejun Son, Joon Ho Moon, Sung Hee Choi, Nam H. Cho, Soo Heon Kwak, Hak Chul Jang
Endocrinol Metab. 2024;39(5):701-710. doi: 10.3803/EnM.2024.1974.
Reference
-
1. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019; 5:47.
Article2. Fu J, Retnakaran R. The life course perspective of gestational diabetes: an opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine. 2022; 45:101294.
Article3. O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964; 13:278–85.4. Saravanan P; Diabetes in Pregnancy Working Group; Maternal Medicine Clinical Study Group; Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020; 8:793–800.
Article5. Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care. 2008; 31:1275–81.
Article6. Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. Risk of early progression to prediabetes or diabetes in women with recent gestational dysglycaemia but normal glucose tolerance at 3-month postpartum. Clin Endocrinol (Oxf). 2010; 73:476–83.7. Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of β-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care. 2014; 37:3262–9.8. Retnakaran R, Shah BR. Abnormal screening glucose challenge test in pregnancy and future risk of diabetes in young women. Diabet Med. 2009; 26:474–7.9. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005; 115:485–91.
Article10. Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001; 86:989–93.
Article11. Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Beta-cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care. 2010; 33:1798–804.12. Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010; 59:2625–30.
Article13. Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care. 2010; 33:396–401.
Article14. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009; 373:1773–9.
Article15. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and metaanalysis. BMJ. 2020; 369:m1361.
Article16. Beharier O, Sergienko R, Kessous R, Szaingurten-Solodkin I, Walfisch A, Shusterman E, et al. Gestational diabetes mellitus is a significant risk factor for long-term ophthalmic morbidity. Arch Gynecol Obstet. 2017; 295:1477–82.
Article17. Beharier O, Shoham-Vardi I, Pariente G, Sergienko R, Kessous R, Baumfeld Y, et al. Gestational diabetes mellitus is a significant risk factor for long-term maternal renal disease. J Clin Endocrinol Metab. 2015; 100:1412–6.18. Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care. 2017; 40:101–8.
Article19. Rawal S, Olsen SF, Grunnet LG, Ma RC, Hinkle SN, Granstrom C, et al. Gestational diabetes mellitus and renal function: a prospective study with 9- to 16-year follow-up after pregnancy. Diabetes Care. 2018; 41:1378–84.
Article20. Kew S, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Postpartum microalbuminuria after gestational diabetes: the impact of current glucose tolerance status. J Clin Endocrinol Metab. 2015; 100:1130–6.
Article21. De Souza LR, Berger H, Retnakaran R, Vlachou PA, Maguire JL, Nathens AB, et al. Non-alcoholic fatty liver disease in early pregnancy predicts dysglycemia in mid-pregnancy: prospective study. Am J Gastroenterol. 2016; 111:665–70.
Article22. Forbes S, Taylor-Robinson SD, Patel N, Allan P, Walker BR, Johnston DG. Increased prevalence of non-alcoholic fatty liver disease in European women with a history of gestational diabetes. Diabetologia. 2011; 54:641–7.23. Ajmera VH, Gunderson EP, VanWagner LB, Lewis CE, Carr JJ, Terrault NA. Gestational diabetes mellitus is strongly associated with non-alcoholic fatty liver disease. Am J Gastroenterol. 2016; 111:658–64.
Article24. Foghsgaard S, Andreasen C, Vedtofte L, Andersen ES, Bahne E, Strandberg C, et al. Nonalcoholic fatty liver disease is prevalent in women with prior gestational diabetes mellitus and independently associated with insulin resistance and waist circumference. Diabetes Care. 2017; 40:109–16.25. Mehmood S, Margolis M, Ye C, Maple-Brown L, Hanley AJ, Connelly PW, et al. Hepatic fat and glucose tolerance in women with recent gestational diabetes. BMJ Open Diabetes Res Care. 2018; 6:e000549.
Article26. Retnakaran R, Luo J, Shah BR. Gestational diabetes in young women predicts future risk of serious liver disease. Diabetologia. 2019; 62:306–10.
Article27. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019; 62:905–14.28. Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG. 2014; 121:1530–6.29. Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014; 180:41–4.30. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of history of gestational diabetes with longterm cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017; 177:1735–42.
Article31. McKenzie-Sampson S, Paradis G, Healy-Profitos J, St-Pierre F, Auger N. Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol. 2018; 55:315–22.32. Retnakaran R, Shah BR. Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2019; 7:378–84.33. Yu Y, Soohoo M, Sorensen HT, Li J, Arah OA. Gestational diabetes mellitus and the risks of overall and type-specific cardiovascular diseases: a population- and sibling-matched cohort study. Diabetes Care. 2022; 45:151–9.34. Echouffo-Tcheugui JB, Guan J, Retnakaran R, Shah BR. Gestational diabetes and incident heart failure: a cohort study. Diabetes Care. 2021; 44:2346–52.
Article35. Verma A, Boney CM, Tucker R, Vohr BR. Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2002; 87:3227–35.36. Lauenborg J, Mathiesen E, Hansen T, Glumer C, Jorgensen T, Borch-Johnsen K, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005; 90:4004–10.37. Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010; 95:670–7.
Article38. Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of low-density-lipoprotein cholesterol and apolipoprotein B in young women: implications for future cardiovascular risk. J Clin Endocrinol Metab. 2010; 95:4345–53.39. Tranidou A, Dagklis T, Tsakiridis I, Siargkas A, Apostolopoulou A, Mamopoulos A, et al. Risk of developing metabolic syndrome after gestational diabetes mellitus: a systematic review and meta-analysis. J Endocrinol Invest. 2021; 44:1139–49.
Article40. Tisi DK, Burns DH, Luskey GW, Koski KG. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care. 2011; 34:139–44.41. Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016; 39:982–7.
Article42. Retnakaran R. The insulin-like growth factor axis: a new player in gestational diabetes mellitus? Diabetes. 2016; 65:3246–8.43. Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. Am J Epidemiol. 2010; 172:1131–43.
Article44. Hedderson MM, Darbinian JA, Quesenberry CP, Ferrara A. Pregravid cardiometabolic risk profile and risk for gestational diabetes mellitus. Am J Obstet Gynecol. 2011; 205:55.45. Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011; 22:724–30.
Article46. Retnakaran R, Shah BR. Divergent trajectories of cardiovascular risk factors in the years before pregnancy in women with and without gestational diabetes mellitus: a population-based study. Diabetes Care. 2020; 43:2500–8.
Article47. Retnakaran R, Shah BR. Impact of pregnancy on the trajectories of cardiovascular risk factors in women with and without gestational diabetes. Diabetes Obes Metab. 2021; 23:2364–73.
Article48. Retnakaran R. Diabetes in pregnancy 100 years after the discovery of insulin: hot topics and open questions to be addressed in the coming years. Metabolism. 2021; 119:154772.49. Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990; 301:1111.
Article50. Moore TR. A comparison of amniotic fluid fetal pulmonary phospholipids in normal and diabetic pregnancy. Am J Obstet Gynecol. 2002; 186:641–50.
Article51. Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong C, et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010; 126:e1545–52.
Article52. Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Clin Perinatol. 2007; 34:611–26.
Article53. Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020; 43:2983–90.
Article54. Pedersen J. Diabetes mellitus and pregnancy: present status of the hyperglycaemia: hyperinsulinism theory and the weight of the newborn baby. Postgrad Med J. 1971; Suppl:66–7.55. Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003; 189:1698–704.
Article56. Crume TL, Shapiro AL, Brinton JT, Glueck DH, Martinez M, Kohn M, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab. 2015; 100:1672–80.57. Pirkola J, Pouta A, Bloigu A, Hartikainen AL, Laitinen J, Jarvelin MR, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010; 33:1115–21.
Article58. Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019; 42:372–80.59. Lowe WL Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018; 320:1005–16.60. Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003; 111:e221–6.
Article61. Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987; 10:76–80.
Article62. Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991; 40 Suppl 2:121–5.
Article63. Gomes D, von Kries R, Delius M, Mansmann U, Nast M, Stubert M, et al. Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: an interim analysis from a longitudinal mother-child cohort study. PLoS Med. 2018; 15:e1002681.
Article64. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000; 49:2208–11.
Article65. Josefson JL, Catalano PM, Lowe WL, Scholtens DM, Kuang A, Dyer AR, et al. The joint associations of maternal BMI and glycemia with childhood adiposity. J Clin Endocrinol Metab. 2020; 105:2177–88.66. Hockett CW, Harrall KK, Moore BF, Starling AP, Bellatorre A, Sauder KA, et al. Persistent effects of in utero overnutrition on offspring adiposity: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia. 2019; 62:2017–24.67. Patro Golab B, Santos S, Voerman E, Lawlor DA, Jaddoe VW, Gaillard R, et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta-analysis. Lancet Child Adolesc Health. 2018; 2:812–21.68. Sauder KA, Hockett CW, Ringham BM, Glueck DH, Dabelea D. Fetal overnutrition and offspring insulin resistance and β-cell function: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabet Med. 2017; 34:1392–9.
Article69. Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019; 42:381–92.70. Wicklow BA, Sellers EA, Sharma AK, Kroeker K, Nickel NC, Philips-Beck W, et al. Association of gestational diabetes and type 2 diabetes exposure in utero with the development of type 2 diabetes in first nations and non-first nations offspring. JAMA Pediatr. 2018; 172:724–31.
Article71. Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM: role of intrauterine environment. Diabetes. 1988; 37:622–8.
Article72. Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB Jr, Liese AD, Vehik KS, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008; 31:1422–6.73. Poulsen P, Esteller M, Vaag A, Fraga MF. The epigenetic basis of twin discordance in age-related diseases. Pediatr Res. 2007; 61(5 Pt 2):38R–42R.
Article74. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008; 105:17046–9.75. Ruchat SM, Houde AA, Voisin G, St-Pierre J, Perron P, Baillargeon JP, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013; 8:935–43.
Article76. Chen P, Piaggi P, Traurig M, Bogardus C, Knowler WC, Baier LJ, et al. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia. 2017; 60:645–55.77. Fradin D, Boelle PY, Belot MP, Lachaux F, Tost J, Besse C, et al. Genome-wide methylation analysis identifies specific epigenetic marks in severely obese children. Sci Rep. 2017; 7:46311.
Article78. Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D, et al. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011; 60:2624–34.79. Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005; 90:3225–9.80. Miranda JO, Cerqueira RJ, Barros H, Areias JC. Maternal diabetes mellitus as a risk factor for high blood pressure in late childhood. Hypertension. 2019; 73:e1–7.81. Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014; 37:436–43.82. Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012; 35:1265–71.83. Sellers EA, Dart AB, McGavock J, Wicklow BA. Cardiovascular comorbidity associated with albuminuria in youth-onset type 2 diabetes: analyses from the iCARE study. Can J Diabetes. 2021; 45:458–65.
Article84. Sellers EA, Hadjiyannakis S, Amed S, Dart AB, Dyck RF, Hamilton J, et al. Persistent albuminuria in children with type 2 diabetes: a canadian paediatric surveillance program study. J Pediatr. 2016; 168:112–7.85. Hoy WE, Ingelfinger JR, Hallan S, Hughson MD, Mott SA, Bertram JF. The early development of the kidney and implications for future health. J Dev Orig Health Dis. 2010; 1:216–33.
Article86. Hokke SN, Armitage JA, Puelles VG, Short KM, Jones L, Smyth IM, et al. Altered ureteric branching morphogenesis and nephron endowment in offspring of diabetic and insulin-treated pregnancy. PLoS One. 2013; 8:e58243.
Article87. Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996; 49:1774–7.88. West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011; 54:504–7.
Article89. Perng W, Hockett CW, Sauder KA, Dabelea D. In utero exposure to gestational diabetes mellitus and cardiovascular risk factors in youth: a longitudinal analysis in the EPOCH cohort. Pediatr Obes. 2020; 15:e12611.
Article90. Grunnet LG, Hansen S, Hjort L, Madsen CM, Kampmann FB, Thuesen AC, et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish national birth cohort. Diabetes Care. 2017; 40:1746–55.
Article91. Guillemette L, Wicklow B, Sellers EA, Dart A, Shen GX, Dolinsky VW, et al. Intrauterine exposure to diabetes and risk of cardiovascular disease in adolescence and early adulthood: a population-based birth cohort study. CMAJ. 2020; 192:E1104–13.
Article92. Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sorensen HT, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019; 367:l6398.
Article93. Kereliuk SM, Dolinsky VW. Recent experimental studies of maternal obesity, diabetes during pregnancy and the developmental origins of cardiovascular disease. Int J Mol Sci. 2022; 23:4467.
Article