Intest Res.
2023 Apr;21(2):244-251. 10.5217/ir.2022.00057.
Concomitant ankylosing spondylitis can increase the risk of biologics or small molecule therapies to control inflammatory bowel disease
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Seoul National University College of Medicine, Seoul, Korea
- 3Division of Gastroenterology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 4Division of Gastroenterology, Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- 5Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2541897
- DOI: http://doi.org/10.5217/ir.2022.00057
Abstract
- Background/Aims
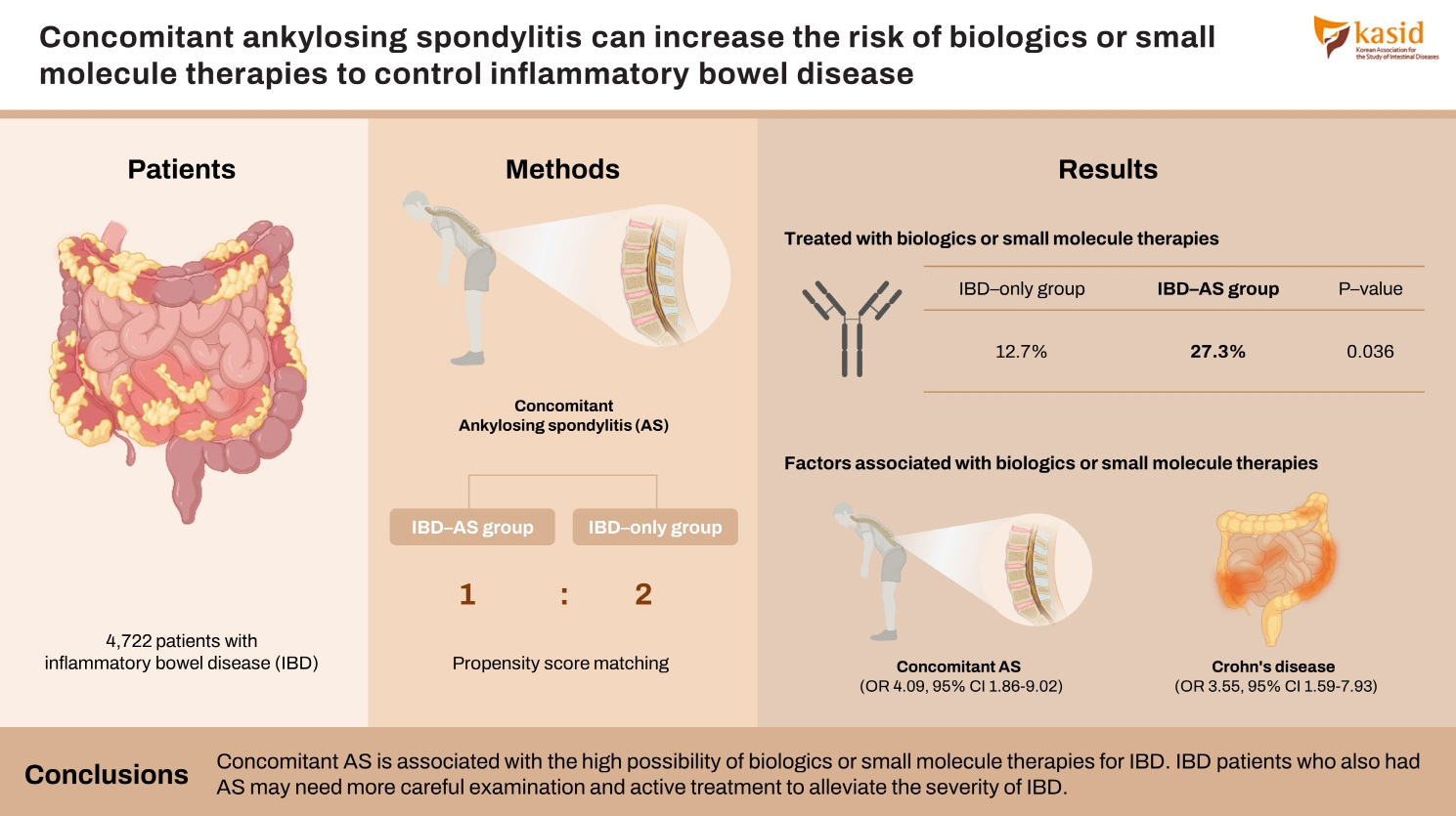
Patients with inflammatory bowel disease (IBD) are diagnosed with ankylosing spondylitis (AS) often. However, the disease course of patients with both IBD and AS is not well understood. This study aims to evaluate the effect of concomitant AS on IBD outcomes.
Methods
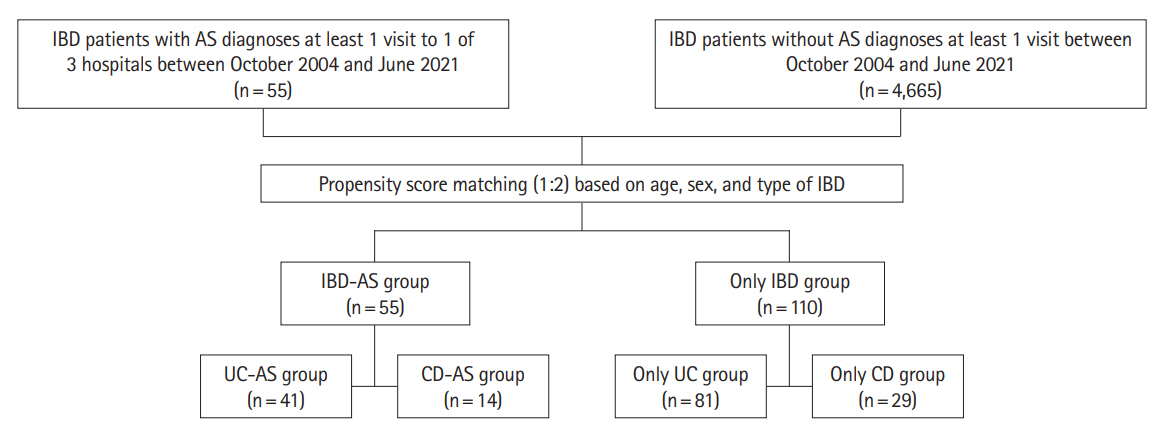
Among the 4,722 patients with IBD who were treated in 3 academic hospitals from 2004 to 2021, 55 were also diagnosed with AS (IBD-AS group). Based on patients’ electronic medical records, the outcomes of IBD in IBD-AS group and IBD group without AS (IBD-only group) were appraised.
Results
The proportion of patients treated with biologics or small molecule therapies was significantly higher in IBD-AS group than the proportion in IBD-only group (27.3% vs. 12.7%, P= 0.036). Patients with both ulcerative colitis and AS had a significantly higher risk of biologics or small molecule therapies than patients with only ulcerative colitis (P< 0.001). For univariable logistic regression, biologics or small molecule therapies were associated with concomitant AS (odds ratio, 4.099; 95% confidence interval, 1.863–9.021; P< 0.001) and Crohn’s disease (odds ratio, 3.552; 95% confidence interval, 1.590–7.934; P= 0.002).
Conclusions
Concomitant AS is associated with the high possibility of biologics or small molecule therapies for IBD. IBD patients who also had AS may need more careful examination and active treatment to alleviate the severity of IBD.
Keyword
Figure
Reference
-
1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007; 448:427–434.
Article2. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007; 369:1379–1390.
Article3. Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: from pathogenesis to treatment. World J Gastroenterol. 2019; 25:2162–2176.
Article4. Essers I, Ramiro S, Stolwijk C, et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology (Oxford). 2015; 54:633–640.
Article5. Van Praet L, Jans L, Carron P, et al. Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann Rheum Dis. 2014; 73:1186–1189.
Article6. Palm O, Moum B, Ongre A, Gran JT. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study). J Rheumatol. 2002; 29:511–515.7. Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014; 53:650–657.
Article8. Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006; 20:451–471.
Article9. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009; 68:777–783.
Article10. Adamina M, Bonovas S, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. 2020; 14:155–168.
Article11. Ross H, Steele SR, Varma M, et al. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2014; 57:5–22.
Article12. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009; 24:63–71.
Article13. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022; 16:2–17.
Article14. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020; 14:4–22.15. Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011; 70:896–904.
Article16. Hamilton L, Barkham N, Bhalla A, et al. BSR and BHPR guideline for the treatment of axial spondyloarthritis (including ankylosing spondylitis) with biologics. Rheumatology (Oxford). 2017; 56:313–316.
Article17. Oh SJ, Shin GY, Soh H, et al. Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res. 2021; 19:323–331.
Article18. Hisamatsu T, Kim HJ, Motoya S, et al. Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI). Intest Res. 2021; 19:386–397.
Article19. Banerjee R, Chuah SW, Hilmi IN, et al. Efficacy and safety of vedolizumab in Crohn’s disease in patients from Asian countries in the GEMINI 2 study. Intest Res. 2021; 19:83–94.
Article20. García MJ, Pascual M, Del Pozo C, et al. Impact of immune-mediated diseases in inflammatory bowel disease and implications in therapeutic approach. Sci Rep. 2020; 10:10731.
Article21. Hiller A, Biedermann L, Fournier N, et al. The appearance of joint manifestations in the Swiss inflammatory bowel disease cohort. PLoS One. 2019; 14:e0211554.
Article22. Ossum AM, Palm Ø, Lunder AK, et al. Ankylosing spondylitis and axial spondyloarthritis in patients with long-term inflammatory bowel disease: results from 20 years of follow-up in the IBSEN study. J Crohns Colitis. 2018; 12:96–104.
Article23. Liu S, Ding J, Wang M, Zhou W, Feng M, Guan W. Clinical features of Crohn disease concomitant with ankylosing spondylitis: a preliminary single-center study. Medicine (Baltimore). 2016; 95:e4267.24. Brown MA. Genetics and the pathogenesis of ankylosing spondylitis. Curr Opin Rheumatol. 2009; 21:318–323.
Article25. Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003; 52:65–70.
Article26. Robinson PC, Leo PJ, Pointon JJ, et al. Exome-wide study of ankylosing spondylitis demonstrates additional shared genetic background with inflammatory bowel disease. NPJ Genom Med. 2016; 1:16008.27. Rezaiemanesh A, Abdolmaleki M, Abdolmohammadi K, et al. Immune cells involved in the pathogenesis of ankylosing spondylitis. Biomed Pharmacother. 2018; 100:198–204.
Article28. Caprioli F, Bosè F, Rossi RL, et al. Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy. Inflamm Bowel Dis. 2013; 19:729–739.
Article29. Lechuga S, Naydenov NG, Feygin A, Cruise M, Ervasti JM, Ivanov AI. Loss of β-cytoplasmic actin in the intestinal epithelium increases gut barrier permeability in vivo and exaggerates the severity of experimental colitis. Front Cell Dev Biol. 2020; 8:588836.
Article30. Zhang L, Wallace CD, Erickson JE, et al. Near infrared readouts offer sensitive and rapid assessments of intestinal permeability and disease severity in inflammatory bowel disease models. Sci Rep. 2020; 10:4696.
Article31. Nighot P, Al-Sadi R, Rawat M, Guo S, Watterson DM, Ma T. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am J Physiol Gastrointest Liver Physiol. 2015; 309:G988–G997.
Article32. Vaile JH, Meddings JB, Yacyshyn BR, Russell AS, Maksymowych WP. Bowel permeability and CD45RO expression on circulating CD20+ B cells in patients with ankylosing spondylitis and their relatives. J Rheumatol. 1999; 26:128–135.33. Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev. 2004; 17:348–369.
Article34. Park JS, Hong JY, Park YS, Han K, Suh SW. Trends in the prevalence and incidence of ankylosing spondylitis in South Korea, 2010-2015 and estimated differences according to income status. Sci Rep. 2018; 8:7694.
Article35. Zhao J, Huang C, Huang H, et al. Prevalence of ankylosing spondylitis in a Chinese population: a systematic review and meta-analysis. Rheumatol Int. 2020; 40:859–872.
Article36. Kwak MS, Cha JM, Lee HH, et al. Emerging trends of inflammatory bowel disease in South Korea: a nationwide population-based study. J Gastroenterol Hepatol. 2019; 34:1018–1026.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pregnancy Outcomes Associated With Biologic Agent Exposure in Patients With Several Rheumatic Diseases and Inflammatory Bowel Diseases
- Primary Biliary Cholangitis with Ankylosing Spondylitis
- Management of inflammatory bowel disease beyond tumor necrosis factor inhibitors: novel biologics and small-molecule drugs
- Increased Fungal Infections while using Emerging Therapies (Biologics and Small-molecule Inhibitors) for Treating Skin Diseases: A Review
- Safety of Biologics and Small Molecules for Inflammatory Bowel Diseases in Organ Transplant Recipients