Korean J Transplant.
2023 Mar;37(1):41-48. 10.4285/kjt.23.0003.
A systematic review and meta-analysis comparing everolimus and calcineurin inhibitors (CNIs) to mycophenolate and CNIs in kidney transplant patients
- Affiliations
-
- 1Section of Nephrology, Department of Medicine, St. Luke’s Medical Center, Quezon City, Philippines
- KMID: 2541311
- DOI: http://doi.org/10.4285/kjt.23.0003
Abstract
- Background
This study compared everolimus and mycophenolate mofetil, each paired with calcineurin inhibitors (CNIs) and used with or without steroids, for maintaining immunosuppression in kidney transplant (KT) patients.
Methods
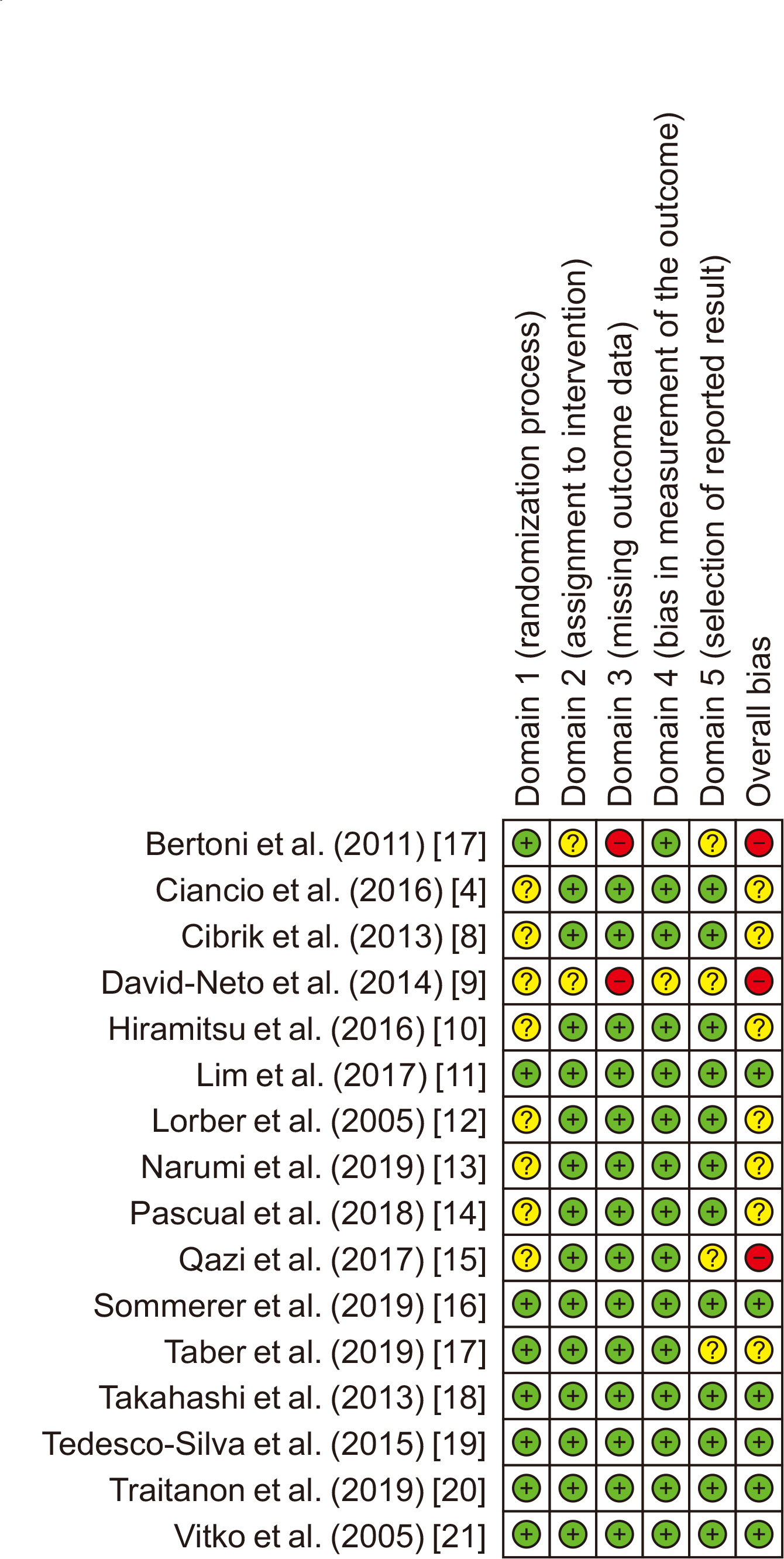
Relevant studies published before August 21, 2022 were retrieved from PubMed, the Cochrane Central Register of Controlled Trials, and the gray literature. The risk of bias was assessed independently using the revised Cochrane risk of bias assessment tool (RoB 2). RevMan ver. 5.4 was used to calculate the risk ratios (RRs) with corresponding 95% confidence intervals (CIs) for biopsy-proven acute rejection, death, and infection. The mean difference (MD) was used to compare the estimated glomerular filtration rate (eGFR) between the groups.
Results
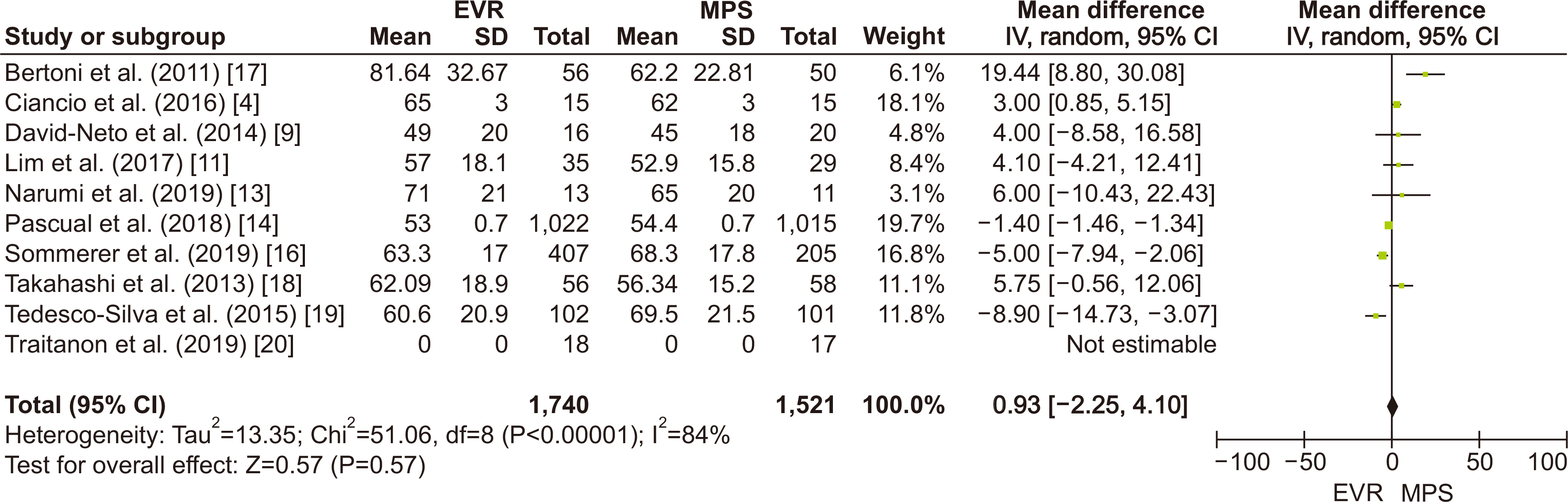
Sixteen randomized controlled trials with a total of 5,403 patients were synthesized to compare everolimus (n=2,763) with mycophenolate (n=2,542) for maintaining post-KT immunosuppression. The meta-analysis showed no significant difference in the risk for biopsy-proven acute rejection (RR=1.12; 95% CI, 0.92–1.35; I2=29%) and death (RR=0.85; 95% CI, 0.63–1.16; I2=0%). The eGFR had no significant difference between the two groups (MD=0.93; 95% CI, −2.25 to 4.1; I2=84%). The risk for any infection was significantly higher in the mycophenolate group than in the everolimus group (RR=0.83; 95% CI, 0.73−0.93; I2=66%).
Conclusions
Our meta-analysis showed that when paired with a CNI, everolimus and mycophenolate had no difference in risk for biopsy-proven acute rejection, death, or increase in eGFR. However, the mycophenolate group exhibited a significantly higher risk of infection.
Figure
Reference
-
1. Malvezzi P, Rostaing L. 2015; The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf. 14:1531–46. DOI: 10.1517/14740338.2015.1083974. PMID: 26329325.2. Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, et al. 2003; Randomized trial of tacrolimus+mycophenolate mofetil or azathioprine versus cyclosporine+mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation. 75:2048–53. DOI: 10.1097/01.TP.0000069831.76067.22. PMID: 12829910.3. Schuurman HJ, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, et al. 1997; SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation. 64:32–5. DOI: 10.1097/00007890-199707150-00007. PMID: 9233697.4. Ciancio G, Tryphonopoulos P, Gaynor JJ, Guerra G, Sageshima J, Roth D, et al. 2016; Pilot randomized trial of tacrolimus/everolimus vs tacrolimus/enteric-coated mycophenolate sodium in adult, primary kidney transplant recipients at a single center. Transplant Proc. 48:2006–10. DOI: 10.1016/j.transproceed.2016.03.048. PMID: 27569936.5. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. 2021; The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 18:e1003583. DOI: 10.1371/journal.pmed.1003583. PMID: 33780438. PMCID: PMC8007028.6. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. 2019; RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 366:l4898. DOI: 10.1136/bmj.l4898. PMID: 31462531.7. Bertoni E, Larti A, Rosso G, Zanazzi M, Di Maria L, Salvadori M. 2011; Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. J Nephrol. 24:613–8. DOI: 10.5301/JN.2011.6247. PMID: 21240873.8. Cibrik D, Silva HT Jr, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, et al. 2013; Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation. 95:933–42. DOI: 10.1097/TP.0b013e3182848e03. PMID: 23422495.9. David-Neto E, Galante N, Altona M, Paula F, Triboni A, Ramos F, et al. 2014; A randomized, prospective study comparing everolimus/low tacrolimus with regular tacrolimus/mps for the elderly renal transplant recipients. Transplantation. 98(Suppl 1):549. DOI: 10.1097/00007890-201407151-01841.10. Hiramitsu T, Okada M, Futamura K, Yamamoto T, Tsujita M, Goto N, et al. 2016; 5-year follow-up of a randomized clinical study comparing everolimus plus reduced-dose cyclosporine with mycophenolate mofetil plus standard-dose cyclosporine in de novo kidney transplantation: retrospective single center assessment. Int Immunopharmacol. 39:192–8. DOI: 10.1016/j.intimp.2016.07.019. PMID: 27491025.11. Lim WH, Russ GR, Wong G, Pilmore H, Kanellis J, Chadban SJ. 2017; The risk of cancer in kidney transplant recipients may be reduced in those maintained on everolimus and reduced cyclosporine. Kidney Int. 91:954–63. DOI: 10.1016/j.kint.2016.11.008. PMID: 28109543.12. Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. 2005; Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation. 80:244–52. DOI: 10.1097/01.TP.0000164352.65613.24. PMID: 16041270.13. Narumi S, Watarai Y, Goto N, Hiramitsu T, Tsujita M, Okada M, et al. 2019; Everolimus-based immunosuppression possibly suppresses mean fluorescence intensity values of de novo donor-specific antibodies after primary kidney transplantation. Transplant Proc. 51:1378–81. DOI: 10.1016/j.transproceed.2019.03.019. PMID: 31056252.14. Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y, et al. 2018; Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol. 29:1979–91. DOI: 10.1681/ASN.2018010009. PMID: 29752413. PMCID: PMC6050928.15. Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR, et al. 2017; Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data. Am J Transplant. 17:1358–69. DOI: 10.1111/ajt.14090. PMID: 27775865.16. Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Witzke O, et al. 2019; An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 96:231–44. DOI: 10.1016/j.kint.2019.01.041. PMID: 31027892.17. Taber DJ, Chokkalingam A, Su Z, Self S, Miller D, Srinivas T. 2019; Randomized controlled trial assessing the impact of everolimus and low-exposure tacrolimus on graft outcomes in kidney transplant recipients. Clin Transplant. 33:e13679. DOI: 10.1111/ctr.13679.18. Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. 2013; Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res. 2:14. DOI: 10.1186/2047-1440-2-14. PMID: 23866828. PMCID: PMC3718642.
Article19. Tedesco-Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes-Freitas T, et al. 2015; Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transplant. 15:2655–64. DOI: 10.1111/ajt.13327. PMID: 25988935.
Article20. Traitanon O, Mathew JM, Shetty A, Bontha SV, Maluf DG, El Kassis Y, et al. 2019; Mechanistic analyses in kidney transplant recipients prospectively randomized to two steroid free regimen-low dose tacrolimus with everolimus versus standard dose tacrolimus with mycophenolate mofetil. PLoS One. 14:e0216300. DOI: 10.1371/journal.pone.0216300. PMID: 31136582. PMCID: PMC6538151.
Article21. Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, et al. 2005; Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 5:2521–30. DOI: 10.1111/j.1600-6143.2005.01063.x. PMID: 16162203.
Article22. Danovitch GM. Handbook of kidney transplantation. Wolters Kluwer;2017.23. Xie X, Jiang Y, Lai X, Xiang S, Shou Z, Chen J. 2015; mTOR inhibitor versus mycophenolic acid as the primary immunosuppression regime combined with calcineurin inhibitor for kidney transplant recipients: a meta-analysis. BMC Nephrol. 16:91. DOI: 10.1186/s12882-015-0078-5. PMID: 26126806. PMCID: PMC4486141.24. Liu J, Liu D, Li J, Zhu L, Zhang C, Lei K, et al. 2017; Efficacy and safety of everolimus for maintenance immunosuppression of kidney transplantation: a meta-analysis of randomized controlled trials. PLoS One. 12:e0170246. DOI: 10.1371/journal.pone.0170246. PMID: 28107397. PMCID: PMC5249216.
Article25. Gao L, Xu F, Cheng H, Liu J. 2018; Comparison of sirolimus combined with tacrolimus and mycophenolate mofetil combined with tacrolimus in kidney transplantation recipients: a meta-analysis. Transplant Proc. 50:3306–13. DOI: 10.1016/j.transproceed.2018.08.056. PMID: 30577200.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of calcineurin inhibitors on rat glioma cells viability
- A Pilot Study of Calcineurin Inhibitors (CNIs) and Steroid Avoidance Immunosuppressive Protocol among Living Donor Kidney Transplant Recipients
- Review of two immunosuppressants: tacrolimus and cyclosporine
- A systematic review and meta-analysis comparing everolimus+CNI with MMF+CNI in kidney transplant
- Long-term clinical outcomes of late conversion to once-daily tacrolimus and sirolimus combination in stable kidney transplant recipients