Cancer Res Treat.
2023 Apr;55(2):570-579. 10.4143/crt.2022.882.
Quality Assessment and Trend for Breast Cancer Treatment Practice across South Korea Based on Nationwide Analysis of Korean Health Insurance Data during 2013-2017
- Affiliations
-
- 1Department of Radiation Oncology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Radiation Oncology, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Surgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 4Department of Radiation Oncology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- KMID: 2541244
- DOI: http://doi.org/10.4143/crt.2022.882
Abstract
- Purpose
Quality assessment of breast cancer treatment in South Korea showed the upward standardization of the grade since 2013, but treatment disparities still have existed. This study analyzed the five year trend between 2013 and 2017 in the assessment of breast cancer treatment practice using the Korean health insurance data.
Materials and Methods
All the medical records including surgery, chemotherapy, and radiotherapy for 7,354 patients a year on average were evaluated. Twenty indices were consisted of one structural, 17 process-related, and 2 result-related factors. We calculated the coefficient of variation (CV) annually to determine the variation in adherence rate of evaluation indices according to the type of institution (advanced vs. general hospital vs. clinic).
Results
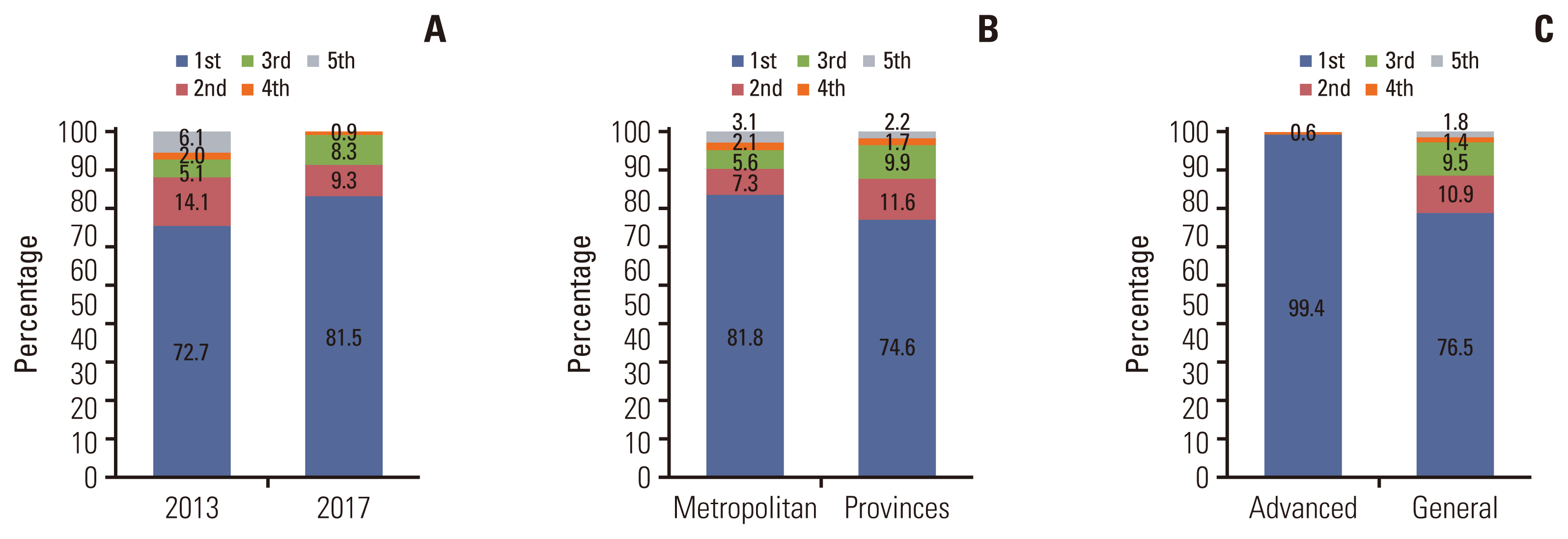
Based on the initial assessment in 2013, 10 out of 20 indicators showed significant variation among the types of institutions with a CV of less than 0.1%. Six of them had a CV decline of less than 0.1%. The CV was still 0.1% or higher in the four indicators, including the composition of professional staff, the implementation of target therapy, the average length of hospital stay, and the hospitalization cost. Regarding the first-grade of assessment, there was a statistically significant relationship between the institution type (p=0.029) and region (metropolitan vs. province, p<0.001).
Conclusion
There were disparities in the structural and systemic treatment factors depending on the institutional type. The quality improvement of the regional institutions and multidisciplinary experts for breast cancer is necessary.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–44.
Article3. Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, et al. Nationwide analysis of treatment patterns for Korean breast cancer survivors using National Health Insurance Service data. J Korean Med Sci. 2018; 33:e276.
Article4. Korean Health Insurance Review and Assessment (HIRA). Evaluation report [Internet]. Wonju: Korean Health Insurance Review and Assessment;2020. [cited 2022 Nov 27]. Available from: https://www.hira.or.kr/ra/eval/asmWrptPopup.do?evlCd=19&pgmid=HIRAA030004000000 .5. Korean Society for Health Equity in Health [Internet]. Seoul: Korean Society for Health Equity in Health;2022. [cited 2022 Nov 27]. Available from: http://healthequity.or.kr/ .6. Kim SH, Kang S, Song MK. Intensity of care at the end of life among older adults in Korea. J Palliat Care. 2018; 33:47–52.
Article7. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009; 20:1319–29.
Article8. Han D, Hogeveen S, Sweet Goldstein M, George R, Brezden-Masley C, Hoch J, et al. Is knowledge translation adequate? A quality assurance study of staging investigations in early stage breast cancer patients. Breast Cancer Res Treat. 2012; 132:1–7.
Article9. Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition: summary document. Oncol Clin Pract. 2008; 4:74–86.10. Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009; 99:488–90.
Article11. Biganzoli L, Marotti L, Hart CD, Cataliotti L, Cutuli B, Kuhn T, et al. Quality indicators in breast cancer care: an update from the EUSOMA working group. Eur J Cancer. 2017; 86:59–81.
Article12. National Quality Forum. National voluntary consensus standards for quality of cancer care. Washington, DC: National Quality Forum;2009.13. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corp;2001.14. Bedeian AG, Mossholder KW. On the use of the coefficient of variation as a measure of diversity. Org Res Methods. 2000; 3:285–97.
Article15. Lunneborg CE. Jonckheere–Terpstra test. Everitt B, Howell D, editors. Encyclopedia of statistics in behavioral science. Hoboken, NJ: John Wiley & Sons;2005.
Article16. Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline update on ovarian suppression. J Clin Oncol. 2016; 34:1689–701.
Article17. Schlam I, Tarantino P, Morganti S, Lynce F, Trapani D, Mayer EL, et al. Emerging targeted therapies for early breast cancer. Drugs. 2022; 82:1437–51.
Article18. Gilligan MA, Neuner J, Zhang X, Sparapani R, Laud PW, Nattinger AB. Relationship between number of breast cancer operations performed and 5-year survival after treatment for early-stage breast cancer. Am J Public Health. 2007; 97:539–44.
Article19. Guller U, Safford S, Pietrobon R, Heberer M, Oertli D, Jain NB. High hospital volume is associated with better outcomes for breast cancer surgery: analysis of 233,247 patients. World J Surg. 2005; 29:994–9.
Article20. Giorgi Rossi P, Lebeau A, Canelo-Aybar C, Saz-Parkinson Z, Quinn C, Langendam M, et al. Recommendations from the European Commission Initiative on Breast Cancer for multigene testing to guide the use of adjuvant chemotherapy in patients with early breast cancer, hormone receptor positive, HER-2 negative. Br J Cancer. 2021; 124:1503–12.
Article21. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. 2014; 15:e279–89.
Article22. Kurebayashi J, Miyoshi Y, Ishikawa T, Saji S, Sugie T, Suzuki T, et al. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: Based on the Breast Cancer Registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer. 2015; 22:235–44.
Article23. Lin CH, Liau JY, Lu YS, Huang CS, Lee WC, Kuo KT, et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol Biomarkers Prev. 2009; 18:1807–14.
Article24. Ou-Yang F, Hsu NC, Juan CH, Huang HI, Moi SH, Chen FM, et al. Breast cancer quality of care in Taiwan in relation to hospital volume: a population-based cohort study. Asia Pac J Clin Oncol. 2015; 11:308–13.
Article25. Mukai H, Higashi T, Sasaki M, Sobue T. Quality evaluation of medical care for breast cancer in Japan. Int J Qual Health Care. 2016; 28:110–3.
Article26. Choi KH, Song JH, Jang HS, Kim SH, Lee JH. Current trends in the quality assessment of colorectal cancer practice and treatment in South Korea during 2012–2017. Cancer Res Treat. 2021; 53:487–96.
Article27. Ramsdale EE, Csik V, Chapman AE, Naeim A, Canin B. Improving quality and value of cancer care for older adults. Am Soc Clin Oncol Educ Book. 2017; 37:383–93.
Article28. Moth EB, Kiely BE, Naganathan V, Martin A, Blinman P. How do oncologists make decisions about chemotherapy for their older patients with cancer? A survey of Australian oncologists. Support Care Cancer. 2018; 26:451–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric Cancer Research using Healthcare Big Data
- Sensitivity of Medical Insurance Claims Data Using Population-based Cancer Registry Data
- Current Trends in the Quality Assessment of Colorectal Cancer Practice and Treatment in South Korea during 2012-2017
- Coronary Event Analysis in Breast Cancer Patients Who Received Breast-Conserving Surgery and PostOperative Radiotherapy: a Korean Nationwide Cohort Study
- Trends and Clinical Characteristics of Next-Generation Sequencing–Based Genetic Panel Tests: An Analysis of Korean Nationwide Claims Data