Cancer Res Treat.
2021 Apr;53(2):487-496. 10.4143/crt.2020.623.
Current Trends in the Quality Assessment of Colorectal Cancer Practice and Treatment in South Korea during 2012-2017
- Affiliations
-
- 1Department of Radiation Oncology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Radiation Oncology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2514930
- DOI: http://doi.org/10.4143/crt.2020.623
Abstract
- Purpose
Colorectal cancer (CRC) is increasing in South Korea due to westernized eating habits and regular health check-ups. The Korean Health Insurance Review and Assessment Service (HIRA) has conducted a national quality assessment of the treatment of CRC. This study examined the quality assessment report of the Korean HIRA and analyzed the status of practice pattern and the epidemiology of CRC in South Korea.
Materials and Methods
The number of subjects was determined based on the number of surgical procedures in each institution during 2012-2017. The institution types were classified according to the number of beds and the composition of oncologic specialists. Twenty-one indicators for diagnosis, chemotherapy, radiotherapy, surgery, pathology, and mortality were analyzed and the interinstitutional variation for each indicator was calculated.
Results
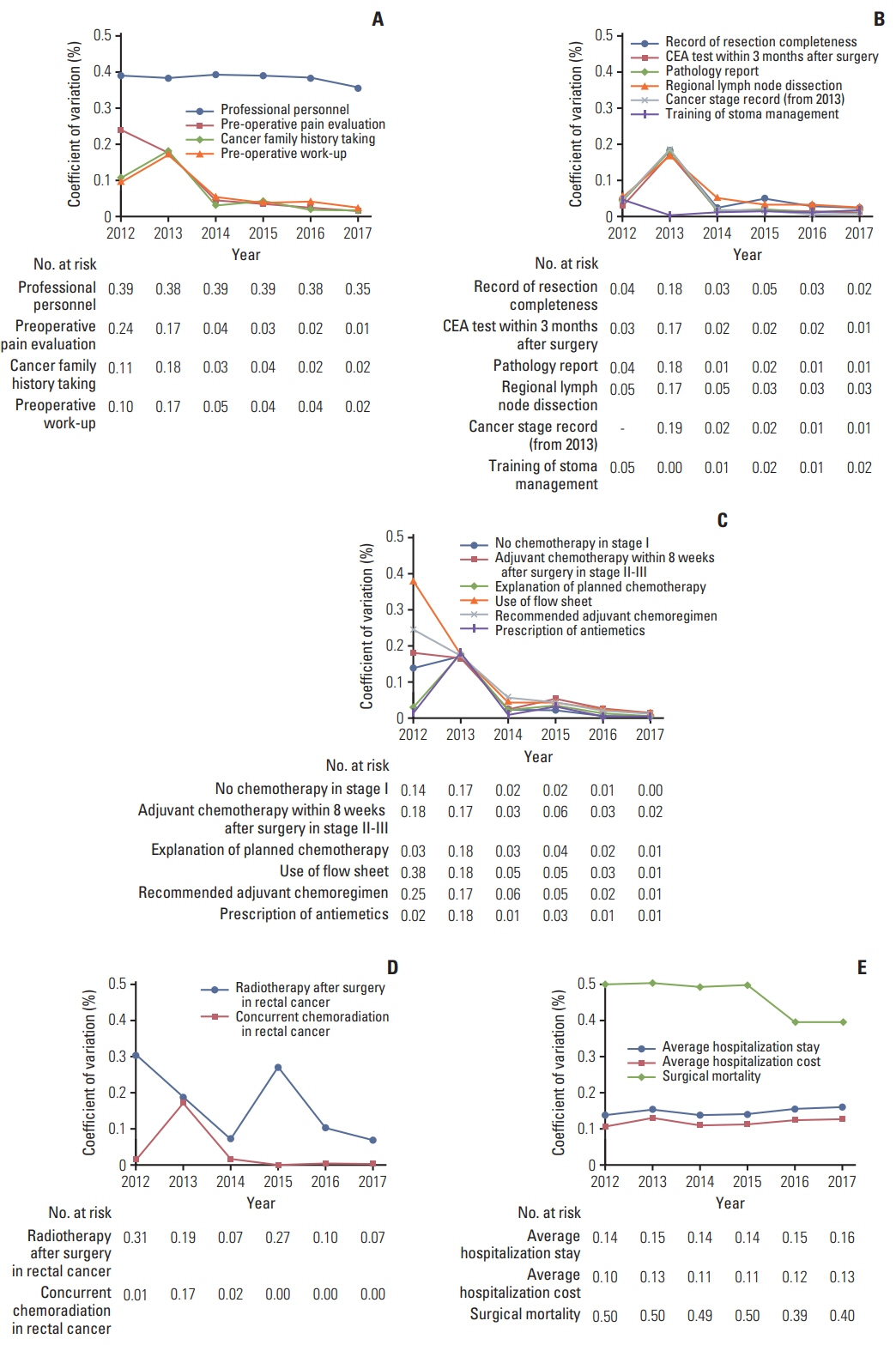
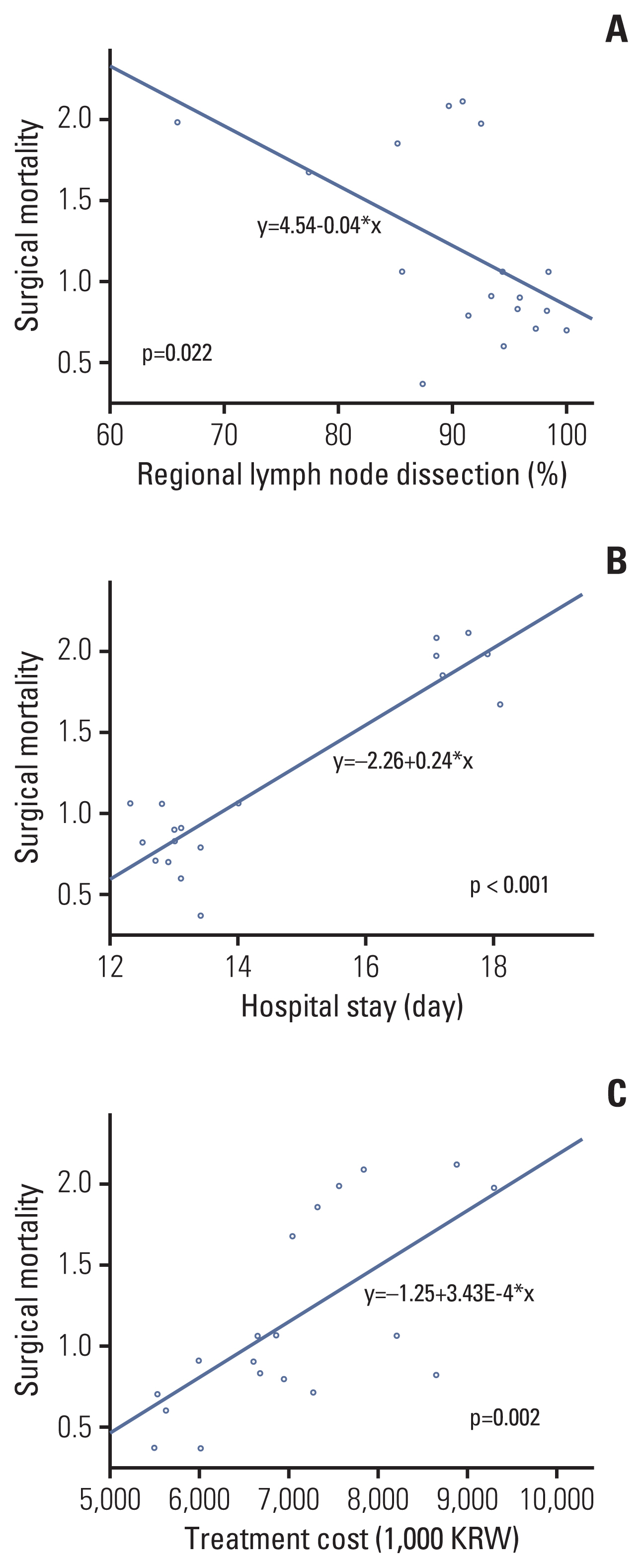
Among 21 evaluation indices, indicators related to medical records, receipt of chemotherapy with a high coefficient of variation of ≥ 0.1% were improved over 6 years until the survey in 2017. In the analysis of indices affecting surgical mortality, the regional lymph node resection and examination rate (p=0.022) showed a negative correlation with surgical mortality. Hospitalization stay (p < 0.001) and hospitalization cost (p=0.002) were positively correlated with surgical mortality.
Conclusion
This study showed that the treatment quality and examination status for CRC in South Korea were appropriate for improving relevant medical records, receipt of chemotherapy, maintaining the quality of treatment, and mortality. These analyses could be the basis for developing an improved quality assessment program worldwide.
Keyword
Figure
Cited by 2 articles
-
Pulmonary Metastasectomy in Colorectal Cancer: A Population-Based Retrospective Cohort Study Using the Korean National Health Insurance Database
Woo Sik Yu, Mi Kyung Bae, Jung Kyu Choi, Young Ki Hong, In Kyu Park
Cancer Res Treat. 2021;53(4):1104-1112. doi: 10.4143/crt.2020.1213.Quality Assessment and Trend for Breast Cancer Treatment Practice across South Korea Based on Nationwide Analysis of Korean Health Insurance Data during 2013–2017
Kyu Hye Choi, Soo-Yoon Sung, Sea-Won Lee, Ye Won Jeon, Sung Hwan Kim, Jong Hoon Lee
Cancer Res Treat. 2023;55(2):570-579. doi: 10.4143/crt.2022.882.
Reference
-
References
1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–50.
Article2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019; 16:713–32.
Article3. Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997; 336:980–7.
Article4. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001; 345:638–46.
Article5. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.
Article6. van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon and rectum. Eur J Cancer. 2014; 50:1.7. Banks I, Weller D, Ungan M, Selby P, Aapro M, Beishon M, et al. ECCO essential requirements for quality cancer care: primary care. Crit Rev Oncol Hematol. 2019; 142:187–99.
Article8. De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5: a population-based study. Lancet Oncol. 2014; 15:23–34.9. Malin JL, Asch SM, Kerr EA, McGlynn EA. Evaluating the quality of cancer care: development of cancer quality indicators for a global quality assessment tool. Cancer. 2000; 88:701–7.10. McGory ML, Shekelle PG, Ko CY. Development of quality indicators for patients undergoing colorectal cancer surgery. J Natl Cancer Inst. 2006; 98:1623–33.
Article11. Dixon E, Armstrong C, Maddern G, Sutherland F, Hemming A, Wei A, et al. Development of quality indicators of care for patients undergoing hepatic resection for metastatic colorectal cancer using a Delphi process. J Surg Res. 2009; 156:32–8.
Article12. Ludt S, Urban E, Eckardt J, Wache S, Broge B, Kaufmann-Kolle P, et al. Evaluating the quality of colorectal cancer care across the interface of healthcare sectors. PLoS One. 2013; 8:e60947.
Article13. Compton CC. Key issues in reporting common cancer specimens: problems in pathologic staging of colon cancer. Arch Pathol Lab Med. 2006; 130:318–24.
Article14. Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009; 133:1539–51.
Article15. Shen SS, Haupt BX, Ro JY, Zhu J, Bailey HR, Schwartz MR. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med. 2009; 133:781–6.
Article16. Yang L, Xiong Z, Xie Q, He W, Liu S, Kong P, et al. Prognostic value of total number of lymph nodes retrieved differs between left-sided colon cancer and right-sided colon cancer in stage III patients with colon cancer. BMC Cancer. 2018; 18:558.
Article17. Chen YJ, Yeh ST, Kao PS, Ou LH, Lin CS. A reappraisal of lymph node dissection in colorectal cancer during primary surgical resection. World J Surg Oncol. 2020; 18:97.
Article18. Aghili M, Khalili N, Khalili N, Babaei M, Farhan F, Haddad P, et al. Short-course versus long-course neoadjuvant chemoradiotherapy in patients with rectal cancer: preliminary results of a randomized controlled trial. Radiat Oncol J. 2020; 38:119–28.
Article19. AlQudah M, Salmo E, Haboubi N. The effect of radiotherapy on rectal cancer: a histopathological appraisal and prognostic indicators. Radiat Oncol J. 2020; 38:77–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Influencing Supportive Care Needs of Colorectal Cancer Survivors
- Trends in Colorectal Cancer Incidence in Daejeon and Chungcheongnam-do, South Korea (2000-2012)
- Changing trends in colorectal cancer in the Republic of Korea: contrast with Japan
- Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002-2012
- Recent Research Trends in Meta-analysis