Cancer Res Treat.
2024 Jan;56(1):27-36. 10.4143/crt.2023.844.
Trends and Clinical Characteristics of Next-Generation Sequencing–Based Genetic Panel Tests: An Analysis of Korean Nationwide Claims Data
- Affiliations
-
- 1Department of Pathology, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 2Department of Policy Research Affairs, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 3Department of Oncology, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 4Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2550320
- DOI: http://doi.org/10.4143/crt.2023.844
Abstract
- Purpose
In the modern era of precision medicine, next-generation sequencing (NGS) is employed for a variety of clinical purposes. The aim of this study was to investigate the trends and clinical characteristics of NGS testing in South Korea.
Materials and Methods
This nationwide, population-based, retrospective cohort study examined National Health Insurance Service claims data from 2017 to 2021 for NGS and from 2008 to 2021 for gene-targeted anticancer drugs.
Results
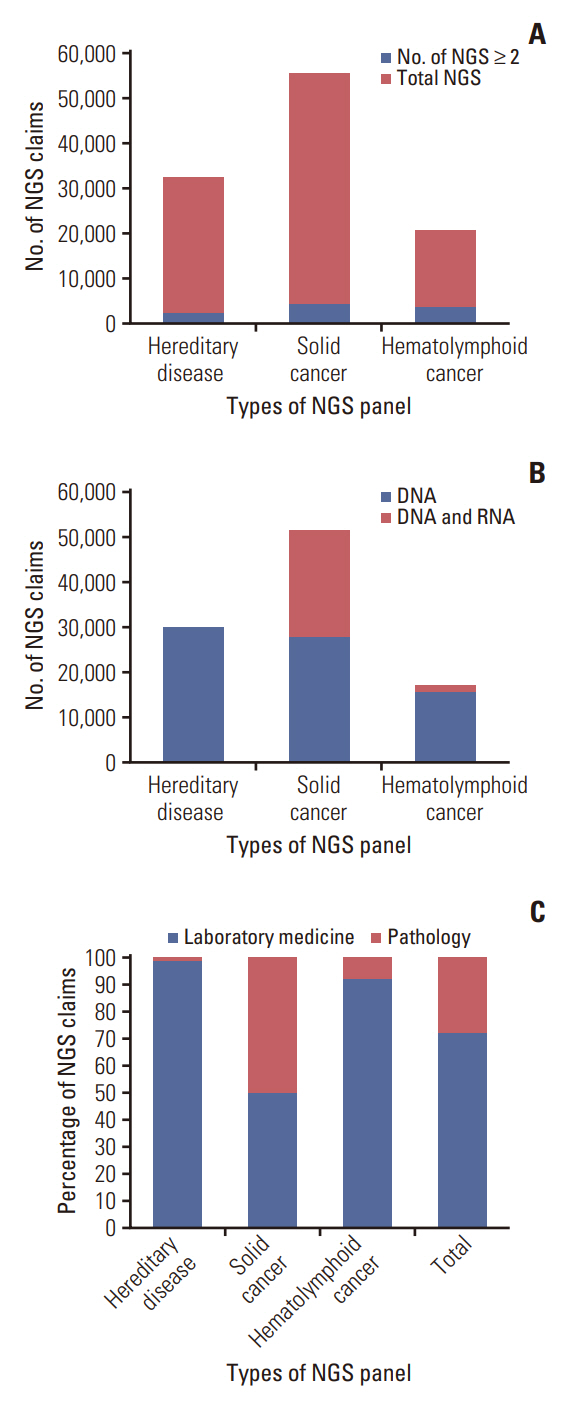
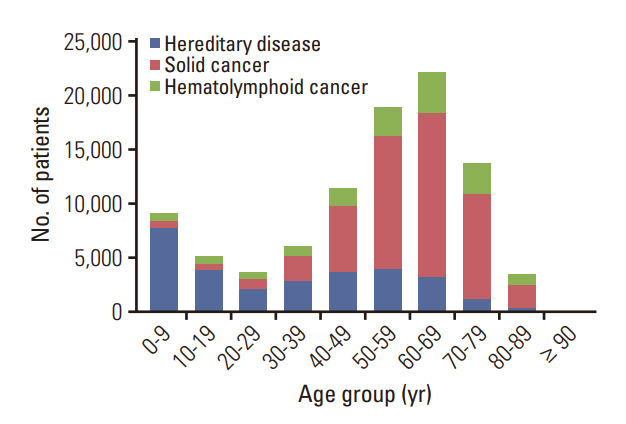
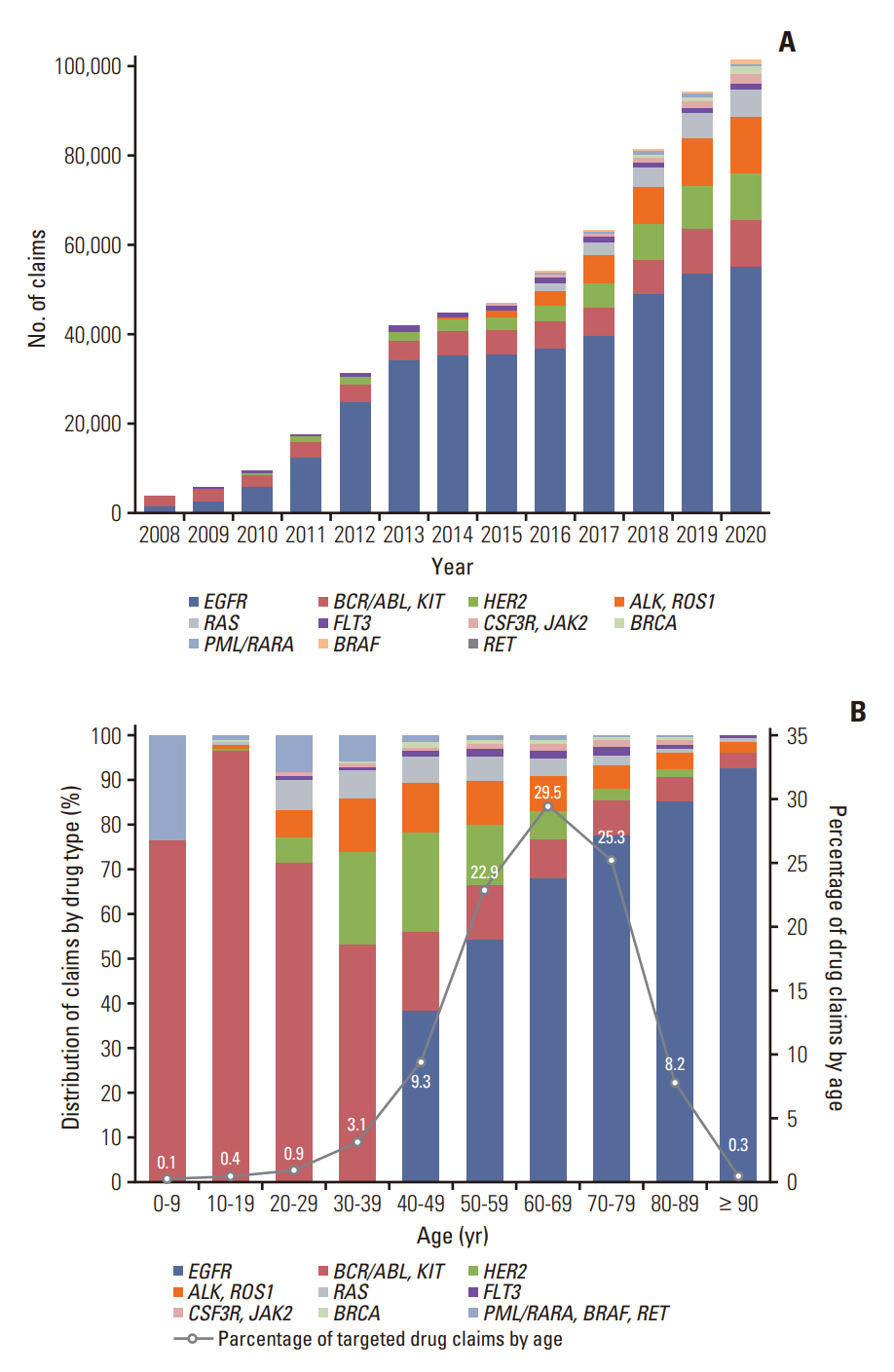
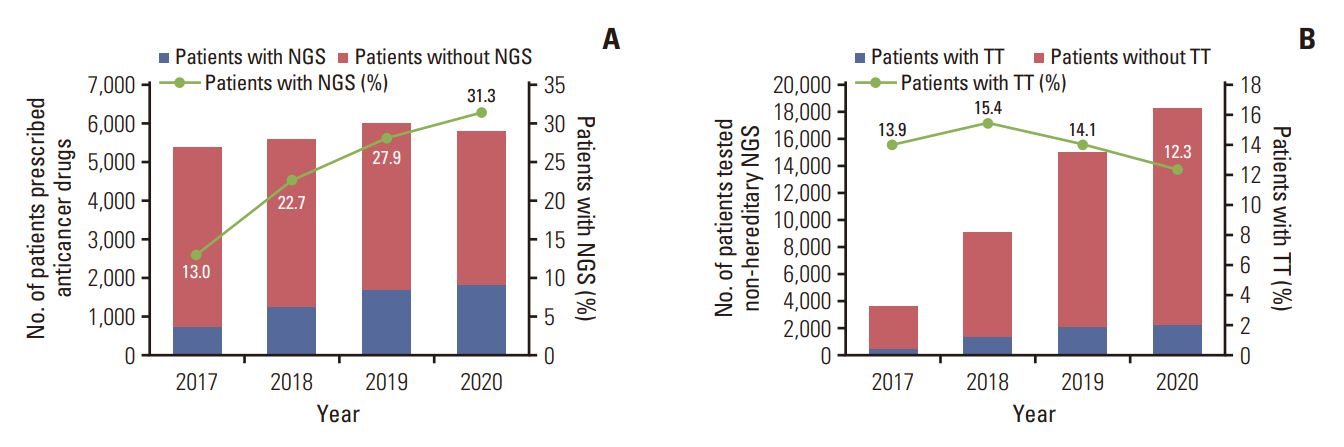
Among the total 98,748 claims, there were 51,407 (52.1%) solid cancer panels, 30,173 (30.5%) hereditary disease panels, and 17,168 (17.4%) hematolymphoid cancer panels. The number of annual claims showed a persistent upward trend, exhibiting a 5.4-fold increase, from 5,436 in 2017 to 29,557 in 2021. In the solid cancer panel, colorectal cancer was the most common (19.2%), followed by lung cancer (18.8%). The annual claims for targeted cancer drugs have increased 25.7-fold, from 3,932 in 2008 to 101,211 in 2020. Drugs for the treatment of lung cancer accounted for 488,819 (71.9%) claims. The number of patients who received non-hereditary NGS testing has substantially increased, and among them, the count of patients prescribed targeted anticancer drugs consistently rose from 508 (13.9%) in 2017 to 2,245 (12.3%) in 2020.
Conclusion
This study highlights the rising nationwide demand for comprehensive genetic testing for disease diagnosis and treatment following NGS reimbursement by the National Health Insurance in South Korea, in addition to the need for greater utilization of targeted anticancer drugs.
Keyword
Figure
Cited by 1 articles
-
Harnessing Institutionally Developed Clinical Targeted Sequencing to Improve Patient Survival in Breast Cancer: A Seven-Year Experience
Jiwon Koh, Jinyong Kim, Go-Un Woo, Hanbaek Yi, So Yean Kwon, Jeongmin Seo, Jeong Mo Bae, Jung Ho Kim, Jae Kyung Won, Han Suk Ryu, Yoon Kyung Jeon, Dae-Won Lee, Miso Kim, Tae-Yong Kim, Kyung-Hun Lee, Tae-You Kim, Jee-Soo Lee, Moon-Woo Seong, Sheehyun Kim, Sungyoung Lee, Hongseok Yun, Myung Geun Song, Jaeyong Choi, Jong-Il Kim, Seock-Ah Im
Cancer Res Treat. 2025;57(2):443-456. doi: 10.4143/crt.2024.296.
Reference
-
References
1. Gagan J, Van Allen EM. Next-generation sequencing to guide cancer therapy. Genome Med. 2015; 7:80.2. Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008; 26:1135–45.3. Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER. The next-generation sequencing revolution and its impact on genomics. Cell. 2013; 155:27–38.4. Nagahashi M, Shimada Y, Ichikawa H, Kameyama H, Takabe K, Okuda S, et al. Next generation sequencing-based gene panel tests for the management of solid tumors. Cancer Sci. 2019; 110:6–15.5. Chakravarty D, Johnson A, Sklar J, Lindeman NI, Moore K, Ganesan S, et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. J Clin Oncol. 2022; 40:1231–58.6. Centers for Medicare & Medicaid Services (CMS). CMS finalizes coverage of next generation sequencing tests, ensuring access for cancer patients [Internet]. Baltimore, MD: Centers for Medicare & Medicaid Services;2018 [cited 2023 Mar 1]. Available from: https://www.cms.gov/newsroom/press-releases/cms-finalizes-coverage-next-generation-sequencing-test-sensuring-enhanced-access-cancer-patients.7. Horgan D, Curigliano G, Riess O, Hofman P, Buttner R, Conte P, et al. Identifying the steps required to effectively implement next-generation sequencing in oncology at a national level in Europe. J Pers Med. 2022; 12:72.8. Health Insurance & Assessment Service. 2019 National Health Insurance statistical yearbook. Wonju: Health Insurance & Assessment Service;2020.9. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017; 46:348–55.10. Korean Statistical Information Service. 2021 Statistical yearbook on the usage of medical services by region [Internet]. Daejeon: Statistics Korea;2022 [cited 2023 Mar 2]. Available from: https://kosis.kr.11. National Health Insurance Service. 2020 National Health Insurance statistical yearbook. Wonju: Health Insurance & Assessment Service;2021.12. Onecha E, Rapado I, Luz Morales M, Carreno-Tarragona G, Martinez-Sanchez P, Gutierrez X, et al. Monitoring of clonal evolution of acute myeloid leukemia identifies the leukemia subtype, clinical outcome and potential new drug targets for post-remission strategies or relapse. Haematologica. 2021; 106:2325–33.13. Caplan EO, Wong WB, Ferries E, Hulinsky R, Brown VT, Bordenave K, et al. Novel approach using administrative claims to evaluate trends in oncology multigene panel testing for patients enrolled in medicare advantage health plans. JCO Precis Oncol. 2021; 5:PO.20.00422.14. Luchini C, Bibeau F, Ligtenberg MJ, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019; 30:1232–43.15. Antoniotti C, Korn WM, Marmorino F, Rossini D, Lonardi S, Masi G, et al. Tumour mutational burden, microsatellite instability, and actionable alterations in metastatic colorectal cancer: next-generation sequencing results of TRIBE2 study. Eur J Cancer. 2021; 155:73–84.16. Korea Central Cancer Registry; National Cancer Center. Annual report of cancer statistics in Korea in 2019. Goyang: National Cancer Center;2021.17. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–20.18. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23:1231–51.19. Endris V, Stenzinger A, Pfarr N, Penzel R, Mobs M, Lenze D, et al. NGS-based BRCA1/2 mutation testing of high-grade serous ovarian cancer tissue: results and conclusions of the first international round robin trial. Virchows Arch. 2016; 468:697–705.20. Youn KI. Comparisons of health care utilization patterns and outcome for National Health Insurance and Medical Aid Program cancer patients. J Health Info Stat. 2014; 39:42–60.21. Sheinson DM, Wong WB, Meyer CS, Stergiopoulos S, Lofgren KT, Flores C, et al. Trends in use of next-generation sequencing in patients with solid tumors by race and ethnicity after implementation of the medicare national coverage determination. JAMA Netw Open. 2021; 4:e2138219.22. Pharmaceutical Research and Manufacturers of America. The United States vs. other countries: availability of cancer medicines varies [Internet]. Washington, DC: Pharmaceutical Research and Manufacturers of America;2020 [cited 2023 Mar 7]. Available from: https://phrma.org/resource-center/Topics/Medicare/The-United-States-vs-Other-Countries-Availability-of-Cancer-Medicines-Varies.23. Cho I, Han E. Drug lag and associated factors for approved drugs in Korea compared with the United States. Int J Environ Res Public Health. 2022; 19:2857.24. Park KH, Choi JY, Lim AR, Kim JW, Choi YJ, Lee S, et al. Genomic landscape and clinical utility in Korean advanced pan-cancer patients from prospective clinical sequencing: K-MASTER program. Cancer Discov. 2022; 12:938–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in the Clinical Application of Next-Generation Sequencing
- Genetic tests by next-generation sequencing in children with developmental delay and/or intellectual disability
- Identification of a Heterozygous SPG11 Mutation by Clinical Exome Sequencing in a Patient With Hereditary Spastic Paraplegia: A Case Report
- Principles of Genetic Counseling in the Era of Next-Generation Sequencing
- Genetic evaluation using next-generation sequencing of children with short stature: a single tertiary-center experience