Cancer Res Treat.
2023 Apr;55(2):551-561. 10.4143/crt.2022.272.
Marked Reduction in the Risk of Dementia in Patients with Breast Cancer: A Nationwide Population-Based Cohort Study
- Affiliations
-
- 1Department of Psychiatry, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Institute of Behavioral Sciences in Medicine, Yonsei University College of Medicine, Seoul, Korea
- 3Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea
- 4Division of Breast Surgery, Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2541242
- DOI: http://doi.org/10.4143/crt.2022.272
Abstract
- Purpose
An inverse relationship between cancer and neurodegenerative disease, which presents the possibility of a reduced risk of dementia in cancer patients, has been suggested previously. However, a nationwide longitudinal population-based study of specific types of cancer with due consideration of treatment effects has not been conducted.
Materials and Methods
This nationwide population-based cohort study used data obtained in a 12-year period (January 2007- December 2018) in the Korean National Health Insurance claims database. All female breast cancer patients (age ≥ 50 years) diagnosed between 2009 and 2010 were included after excluding those with physician visits for any cancer during a 2-year period (2007-2008). Patients with senile cataract constituted the control group. The main study outcome was the risk of developing dementia.
Results
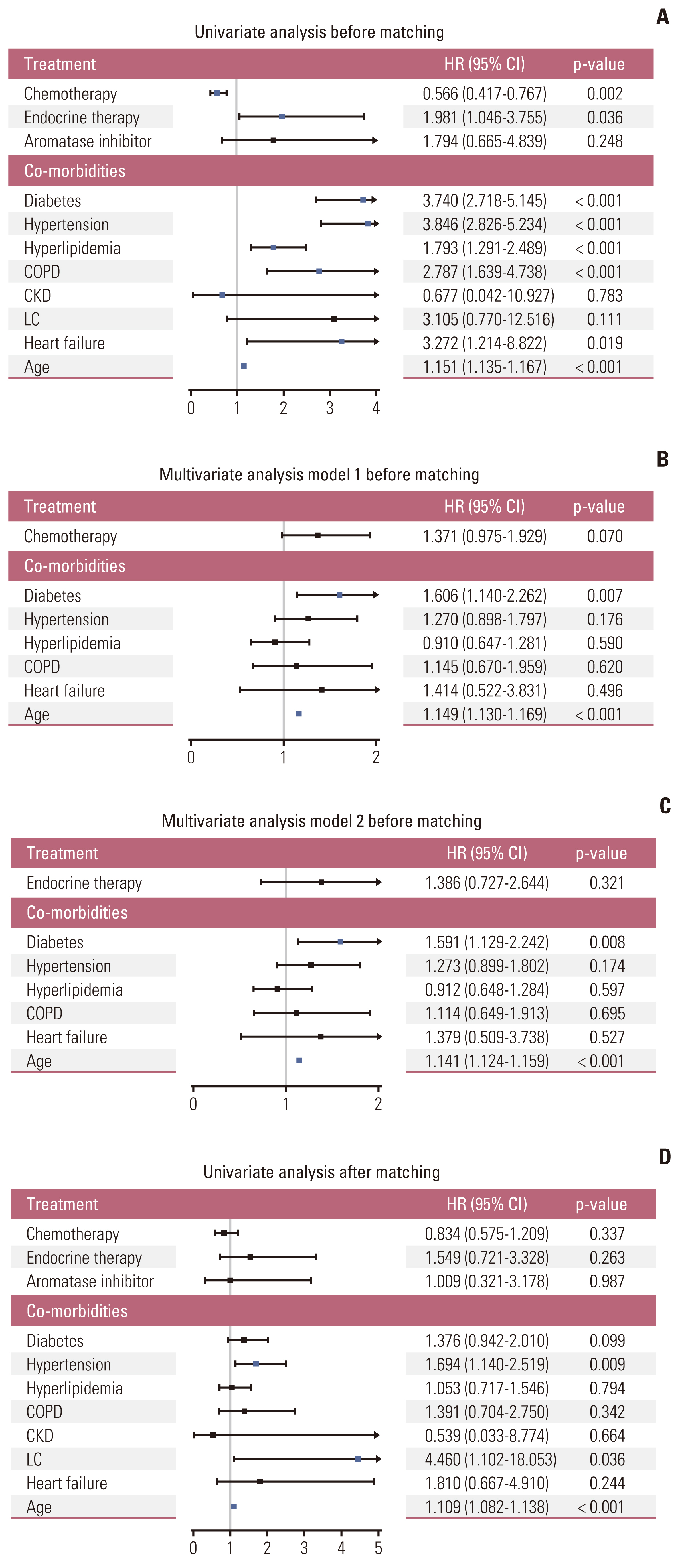
From a total of 90,396 and 85,906 patients with breast cancer and cataract, respectively, patients without behavior codes were excluded. Data for 15,407 breast cancer patients and 7,020 controls were analyzed before matching. After matching for comorbidities and age, either group comprised 2,252 patients. The median follow-up time was 104.1±24.0 months after matching. After matching, breast cancer was a predictor of a lower risk of for dementia (hazard ratio, 0.091; 95% confidence interval, 0.075 to 0.111; p < 0.001). In breast cancer patients, receiving chemotherapy and endocrine therapy did not significantly affect the incidence of dementia.
Conclusion
Breast cancer was associated with a remarkably decreased risk of dementia. The findings strongly suggest an inverse relationship between cancer and neurodegeneration, regardless of the adverse effects of cancer treatment on cognitive function.
Keyword
Figure
Reference
-
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57:43–66.
Article2. Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014; 35:22–31.
Article3. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003; 60:387–92.
Article4. Brunnstrom H, Gustafson L, Passant U, Englund E. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch Gerontol Geriatr. 2009; 49:146–9.
Article5. Wolters FJ, Chibnik LB, Waziry R, Anderson R, Berr C, Beiser A, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer Cohorts Consortium. Neurology. 2020; 95:e519–31.6. Lu H, Wang XD, Shi Z, Yue W, Zhang Y, Liu S, et al. Comparative analysis of cognitive impairment prevalence and its etiological subtypes in a rural area of northern China between 2010 and 2015. Sci Rep. 2019; 9:851.
Article7. Catala-Lopez F, Suarez-Pinilla M, Suarez-Pinilla P, Valderas JM, Gomez-Beneyto M, Martinez S, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother Psychosom. 2014; 83:89–105.
Article8. Yarchoan M, James BD, Shah RC, Arvanitakis Z, Wilson RS, Schneider J, et al. Association of cancer history with Alzheimer’s disease dementia and neuropathology. J Alzheimers Dis. 2017; 56:699–706.
Article9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–74.
Article10. Shafi O. Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: a systematic review. BMC Neurol. 2016; 16:236.
Article11. Nudelman KNH, McDonald BC, Lahiri DK, Saykin AJ. Biological hallmarks of cancer in Alzheimer’s disease. Mol Neurobiol. 2019; 56:7173–87.
Article12. Nixon DW. The inverse relationship between cancer and Alzheimer’s disease: a possible mechanism. Curr Alzheimer Res. 2017; 14:883–93.
Article13. Lin HL, Lin HC, Tseng YF, Chen SC, Hsu CY. Inverse association between cancer and dementia: a population-based registry study in Taiwan. Alzheimer Dis Assoc Disord. 2016; 30:118–22.14. Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012; 344:e1442.
Article15. Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology. 2013; 81:322–8.
Article16. Wang XM, Walitt B, Saligan L, Tiwari AF, Cheung CW, Zhang ZJ. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine. 2015; 72:86–96.
Article17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007; 4:e296.
Article18. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–8.19. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001; 19:585–91.20. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean version of Global Deterioration Scale. J Korean Neurol Assoc. 2002; 20:612–7.21. Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010; 172:1092–7.
Article22. Parsons LS. Performing a 1: N case-control match on propensity score. Cary, NC: SAS Institute; 2004.23. Stagg BC, Ehrlich JR, Choi H, Levine DA. Association of cognitive impairment and dementia with receipt of cataract surgery among community-dwelling medicare beneficiaries. JAMA Ophthalmol. 2019; 137:114–7.
Article24. Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, Kalofonos HP. Either called “chemobrain” or “chemofog,” the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J Pain Symptom Manage. 2011; 41:126–39.
Article25. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011; 95:17–23.
Article26. Xiao Z, Wu W, Zhao Q, Liang X, Luo J, Ding D. Association of glaucoma and cataract with incident dementia: a 5-year follow-up in the Shanghai aging study. J Alzheimers Dis. 2020; 76:529–37.
Article27. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015; 22:1370–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- History of Diabetes Mellitus and Risk of Breast Cancer in Asian Women: A Meta-Epidemiological Analysis of Population-Based Cohort Studies
- Epidemiological characteristics of breast cancer in Koreans
- Age-Period-Cohort Analysis of Female Breast Cancer Mortality in Korea
- Association between Alzheimer's Disease and Cancer Risk in South Korea: an 11-year Nationwide Population-Based Study
- Correlation of Androgen Deprivation Therapy with Cognitive Dysfunction in Patients with Prostate Cancer: A Nationwide Population-Based Study Using the National Health Insurance Service Database