Cancer Res Treat.
2019 Apr;51(2):593-602. 10.4143/crt.2018.119.
Correlation of Androgen Deprivation Therapy with Cognitive Dysfunction in Patients with Prostate Cancer: A Nationwide Population-Based Study Using the National Health Insurance Service Database
- Affiliations
-
- 1Department of Urology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. jaeyoungpark@korea.ac.kr
- 2Korea University School of Medicine, Seoul, Korea.
- KMID: 2464406
- DOI: http://doi.org/10.4143/crt.2018.119
Abstract
- PURPOSE
The purpose of this study was to evaluate the association of androgen deprivation therapy (ADT) with cognitive dysfunction.
MATERIALS AND METHODS
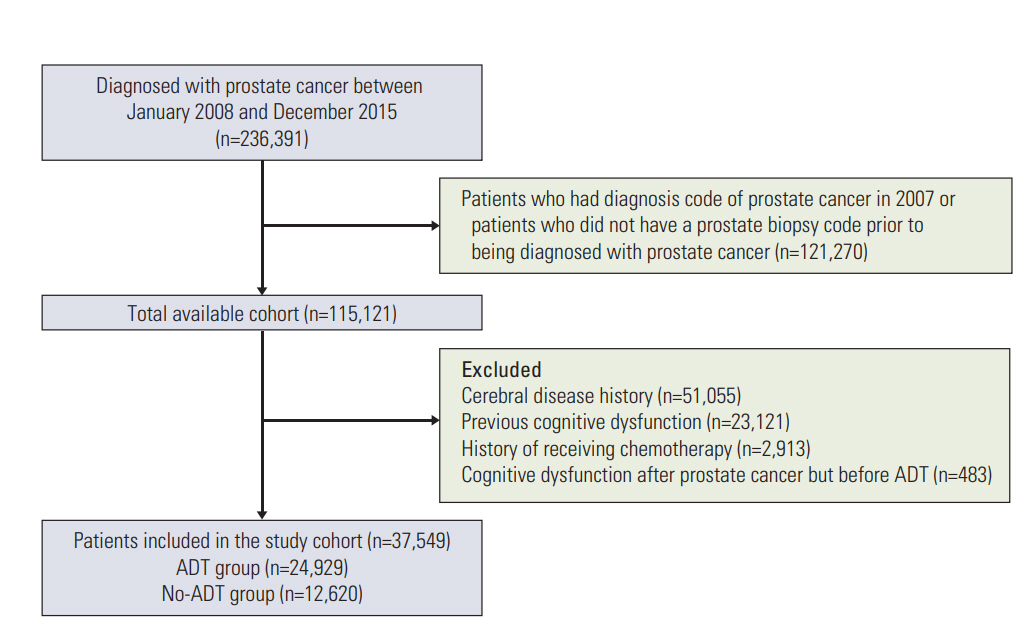
Using the National Health Insurance Service database of the entire Korean adult prostate cancer population (n=236,391), data on ADT and cognitive dysfunction between 2008 and 2015 were analyzed. We excluded patients previously diagnosed with cognitive dysfunction, dementia, or a cerebral event history. We tested the effect of ADT on the risk of cognitive dysfunction using propensity score-matched Cox proportional hazards regression models and Kaplan-Meier survival analysis. Our final cohort comprised of 35,401 individuals with prostate cancer, including 24,567 men (70.6%) who underwent ADT.
RESULTS
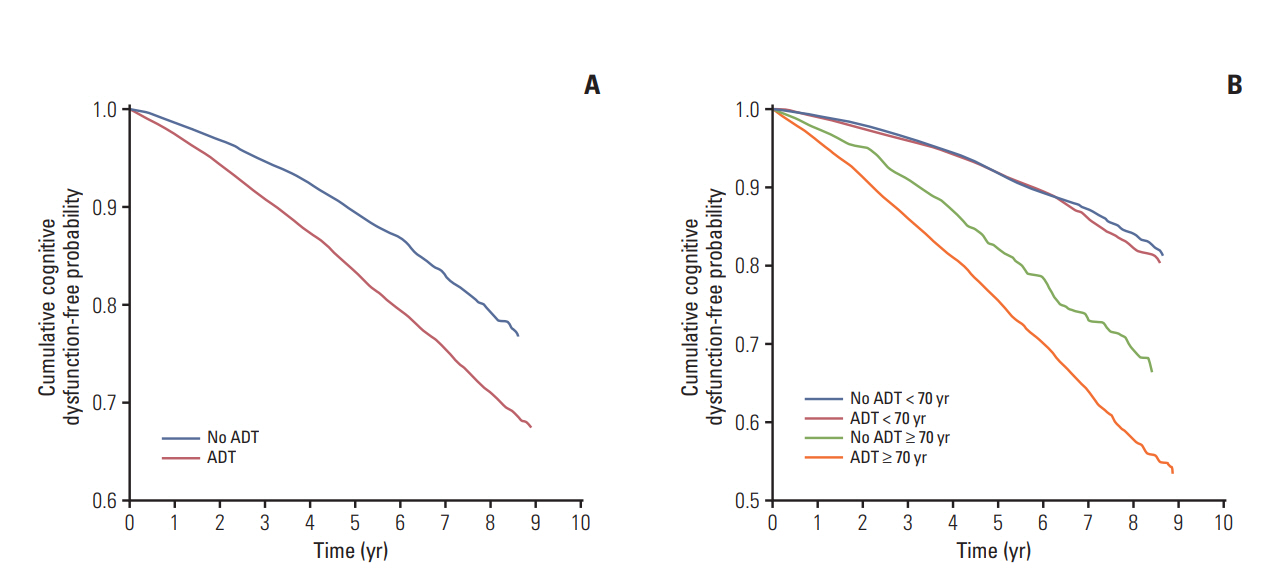
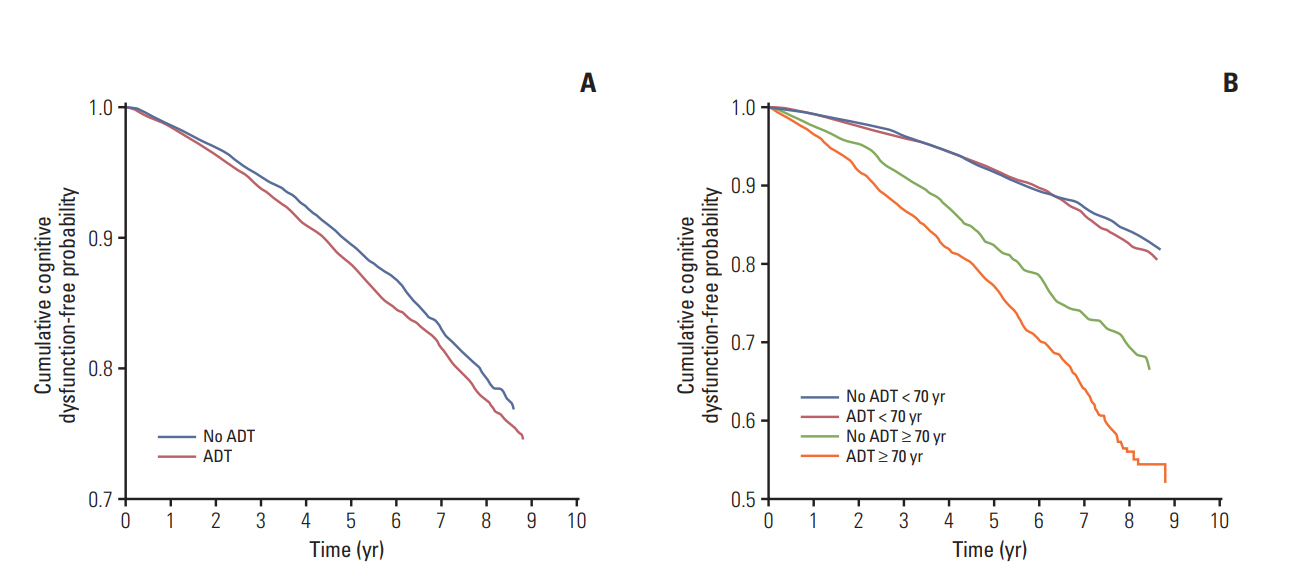
During a mean follow-up period of 4.1 years, 4,741 patients were newly diagnosed with cognitive dysfunction. A statistically significant association was found between ADT and the risk of cognitive dysfunction (hazard ratio, 1.169; p=0.002). Meanwhile, age (≥ 70 years), diabetes, hypertension, cardiovascular history, and peripheral vascular disease were identified as factors that contribute to the increased risk of cognitive dysfunction. In contrast, the use of statins and aspirin was associated with a lower risk of cognitive dysfunction. Kaplan-Meier analysis demonstrated that patients aged 70 years or older who underwent ADT had the lowest cumulative probability of remaining cognitive dysfunction-free (log-rank p < 0.001).
CONCLUSION
Our results revealed an association between the use of ADT for the treatment of prostate cancer and an increased risk of cognitive dysfunction in a nationwide population-based study. This finding should be further evaluated in prospective studies.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002; 360:103–6.
Article2. Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005; 103:1615–24.
Article3. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009; 115:2388–99.4. Choi SM, Kam SC. Metabolic effects of androgen deprivation therapy. Korean J Urol. 2015; 56:12–8.
Article5. Kintzel PE, Chase SL, Schultz LM, O'Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008; 28:1511–22.
Article6. Sharpley CF, Christie DR, Bitsika V. Do hormone treatments for prostate cancer cause anxiety and depression? Int J Clin Oncol. 2014; 19:523–30.
Article7. Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011; 69:322–37.
Article8. Hsu B, Cumming RG, Waite LM, Blyth FM, Naganathan V, Le Couteur DG, et al. Longitudinal relationships between reproductive hormones and cognitive decline in older men: the Concord Health and Ageing in Men Project. J Clin Endocrinol Metab. 2015; 100:2223–30.
Article9. Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, et al. Androgen deprivation therapy and future Alzheimer's disease risk. J Clin Oncol. 2016; 34:566–71.
Article10. Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH. Association Between androgen deprivation therapy and risk of dementia. JAMA Oncol. 2017; 3:49–55.
Article11. Khosrow-Khavar F, Rej S, Yin H, Aprikian A, Azoulay L. Androgen deprivation therapy and the risk of dementia in patients with prostate cancer. J Clin Oncol. 2017; 35:201–7.
Article12. Kao LT, Lin HC, Chung SD, Huang CY. No increased risk of dementia in patients receiving androgen deprivation therapy for prostate cancer: a 5-year follow-up study. Asian J Androl. 2017; 19:414–7.
Article13. McHugh DJ, Root JC, Nelson CJ, Morris MJ. Androgen-deprivation therapy, dementia, and cognitive dysfunction in men with prostate cancer: How much smoke and how much fire? Cancer. 2018; 124:1326–34.
Article14. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. 2014; 38:395–403.
Article15. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007; 7:192–201.
Article16. Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005; 55:241–52.17. Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999; 84:3681–5.
Article18. Muller M, Aleman A, Grobbee DE, de Haan EH, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men: is there an optimal level? Neurology. 2005; 64:866–71.19. Hyde Z, Flicker L, Almeida OP, McCaul KA, Jamrozik K, Hankey GJ, et al. Higher luteinizing hormone is associated with poor memory recall: the health in men study. J Alzheimers Dis. 2010; 19:943–51.
Article20. Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009; 30:239–58.
Article21. Chao HH, Uchio E, Zhang S, Hu S, Bednarski SR, Luo X, et al. Effects of androgen deprivation on brain function in prostate cancer patients: a prospective observational cohort analysis. BMC Cancer. 2012; 12:371.
Article22. Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002; 90:427–32.
Article23. McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, Cases MG, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014; 22:2271–80.
Article24. Nead KT. Androgen deprivation therapy and dementia: new opportunities and challenges in the big-data era. J Clin Oncol. 2017; 35:3380–1.
Article25. Esme M, Yavuz BB, Yavuz B, Asil S, Tuna Dogrul R, Sumer F, et al. Masked hypertension is associated with cognitive decline in Geriatric Age-Geriatric MASked Hypertension and Cognition (G-MASH-cog) study. J Gerontol A Biol Sci Med Sci. 2018; 73:248–54.
Article26. Dede DS, Yavuz B, Yavuz BB, Cankurtaran M, Halil M, Ulger Z, et al. Assessment of endothelial function in Alzheimer's disease: is Alzheimer's disease a vascular disease? J Am Geriatr Soc. 2007; 55:1613–7.
Article27. Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC 3rd. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995; 131:162–9.
Article28. Palta P, Carlson MC, Crum RM, Colantuoni E, Sharrett AR, Yasar S, et al. Diabetes and cognitive decline in older adults: the Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci. 2017; 73:123–30.
Article29. Chan D, Binks S, Nicholas JM, Frost C, Cardoso MJ, Ourselin S, et al. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. 2017; 16:591–600.
Article30. Wang YP, Wu Y, Li LY, Zheng J, Liu RG, Zhou JP, et al. Aspirin-triggered lipoxin A4 attenuates LPS-induced proinflammatory responses by inhibiting activation of NF-kappaB and MAPKs in BV-2 microglial cells. J Neuroinflammation. 2011; 8:95.
Article31. Veronese N, Stubbs B, Maggi S, Thompson T, Schofield P, Muller C, et al. Low-dose aspirin use and cognitive function in older age: a systematic review and meta-analysis. J Am Geriatr Soc. 2017; 65:1763–8.
Article32. Han JW, So Y, Kim TH, Lee DY, Ryu SH, Kim SY, et al. Prevalence rates of dementia and mild cognitive impairment are affected by the diagnostic parameter changes for neurocognitive disorders in the DSM-5 in a Korean population. Dement Geriatr Cogn Disord. 2017; 43:193–203.
Article33. Kim SH, Kim DH, Kim WS. Long-term care needs of the elderly in Korea and elderly long-term care insurance. Soc Work Public Health. 2010; 25:176–84.
Article34. Lange M, Laviec H, Castel H, Heutte N, Leconte A, Leger I, et al. Impact of new generation hormone-therapy on cognitive function in elderly patients treated for a metastatic prostate cancer: Cog-Pro trial protocol. BMC Cancer. 2017; 17:549.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- How Does Androgen Deprivation Therapy Affect Mental Health including Cognitive Dysfunction in Patients with Prostate Cancer?
- A Nationwide Study of Differences in Surgical Treatment Rates and Oncological Outcomes for Prostate Cancer according to Economic Status and Region
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Current Concepts in Androgen Deprivation Therapy