Cancer Res Treat.
2023 Apr;55(2):468-478. 10.4143/crt.2022.1342.
Optimal Definition of Oligometastasis Showing Survival Benefits of Local Therapies during Tyrosine Kinase Inhibitor Treatment
- Affiliations
-
- 1Department of Hematology and Oncology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2541233
- DOI: http://doi.org/10.4143/crt.2022.1342
Abstract
- Purpose
We aimed to investigate the feasibility of four criteria on oligometastasis (OM) concerning clear survival benefits of local therapy (LT) during tyrosine kinase inhibitor (TKI) treatment in non–small cell lung cancer (NSCLC).
Materials and Methods
This single-center, retrospective study included patients with advanced NSCLC who received LT because of OM during TKI treatment at Asan Medical Center from January 2011 to December 2020. At the application of LT OM was classified according to four criteria: TNM, European Organization for Research and Treatment of Cancer Lung Cancer Group (EORTC-LCG), National Comprehensive Network (NCCN), and ORGAN. We compared survival outcomes between patients with and without OM.
Results
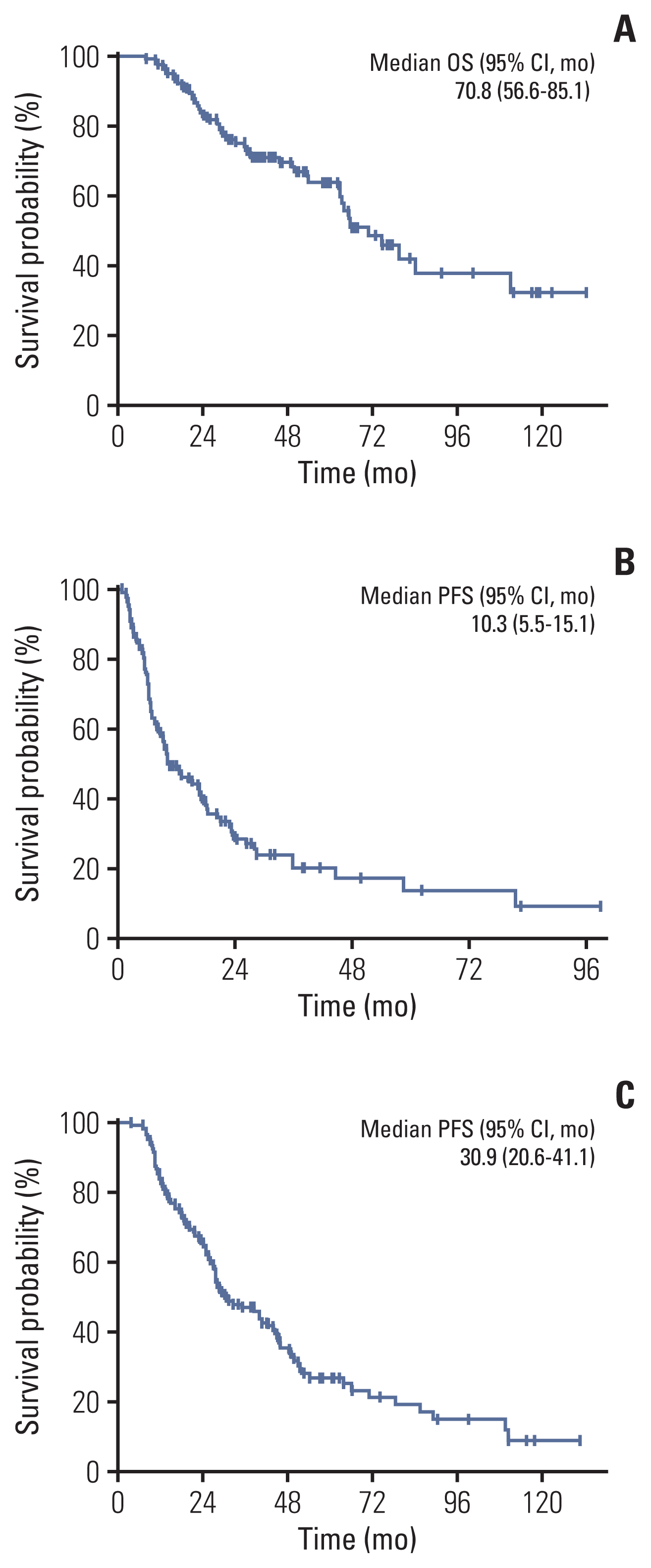
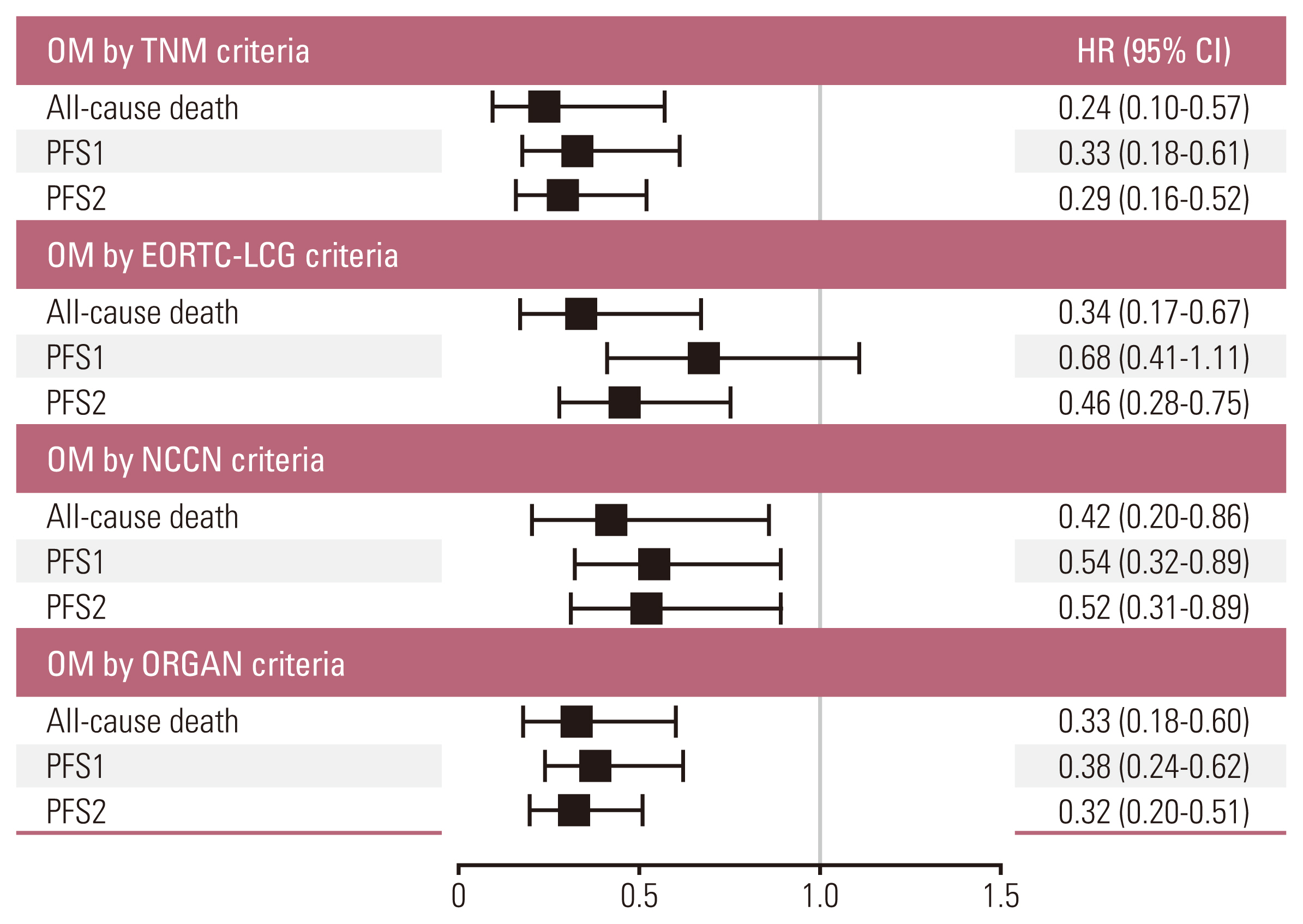
The median overall survival of the 117 patients included in the analysis was 70.8 months (95% confidence interval [CI], 56.6 to 85.1). The patients with OM meeting all four criteria (hazard ratio [HR] with 95% CI of TNM criteria 0.24 with 0.10-0.57; p=0.001, EORTC-LCG criteria 0.34 with 0.17-0.67; p=0.002, NCCN criteria 0.41 with 0.20-0.86; p=0.018 and ORGAN criteria 0.33 with 0.18-0.60; p < 0.001) had significantly longer survival compared with patients who did not after adjusting for confounding factors. Furthermore, increasing the number of extra-thoracic metastatic organs to two or more were independent predictive factors for worse survival outcomes (2 organs: HR, 3.51; 95% CI, 1.01 to 12.14; p=0.048; 3 organs: HR, 4.31; 95% CI, 0.94 to 19.73; p=0.060; 4 organs: HR, 24.47; 95% CI, 5.08 to 117.80; p < 0.001).
Conclusion
Patients with OM defined by all four criteria showed prognostic benefits from LT during TKI therapy.
Figure
Reference
-
References
1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995; 13:8–10.
Article2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021; 71:7–33.
Article3. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014; 11:473–81.
Article4. Li XY, Zhu XR, Zhang CC, Yu W, Zhang B, Shen TL, et al. Analysis of progression patterns and failure sites of patients with metastatic lung adenocarcinoma with EGFR mutations receiving first-line treatment of tyrosine kinase inhibitors. Clin Lung Cancer. 2020; 21:534–44.
Article5. Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018; 13:1383–92.
Article6. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014; 15:346–55.
Article7. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011; 8:378–82.
Article8. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019; 393:2051–8.
Article9. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021; 19:254–66.10. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020; 21:e18–28.
Article11. Blumenthaler AN, Antonoff MB. Classifying oigometastatic non-small cell lung cancer. Cancers (Basel). 2021; 13:4822.12. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017; 151:193–203.
Article13. Dingemans AC, Hendriks LE, Berghmans T, Levy A, Hasan B, Faivre-Finn C, et al. Definition of synchronous oligometastatic non-small cell lung cancer: a consensus report. J Thorac Oncol. 2019; 14:2109–19.14. Correa RJ, Salama JK, Milano MT, Palma DA. Stereotactic body radiotherapy for oligometastasis: opportunities for biology to guide clinical management. Cancer J. 2016; 22:247–56.15. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018; 378:113–25.
Article16. Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017; 28:270–7.
Article17. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012; 366:883–92.
Article18. Naxerova K, Jain RK. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat Rev Clin Oncol. 2015; 12:258–72.
Article19. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011; 147:275–92.
Article20. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003; 3:453–8.
Article21. Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013; 8:346–51.
Article22. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017; 18:1454–66.
Article23. Gan GN, Weickhardt AJ, Scheier B, Doebele RC, Gaspar LE, Kavanagh BD, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014; 88:892–8.
Article24. Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012; 7:1807–14.
Article25. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020; 383:2018–29.
Article26. Lussier YA, Khodarev NN, Regan K, Corbin K, Li H, Ganai S, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012; 7:e50141.
Article27. Uppal A, Wightman SC, Mallon S, Oshima G, Pitroda SP, Zhang Q, et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget. 2015; 6:3540–52.
Article28. Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010; 1805:105–17.
Article29. Warton K, Mahon KL, Samimi G. Methylated circulating tumor DNA in blood: power in cancer prognosis and response. Endocr Relat Cancer. 2016; 23:R157–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Severe Fatigue Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Mechanisms of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance and Strategies to Overcome Resistance in Lung Adenocarcinoma
- Management of Bleeding Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer
- Recent advances in treatments of adult immune thrombocytopenia
- Long-term survival after multimodal treatment involving radiotherapy for huge hepatocellular carcinoma with oligometastasis: a case report