Korean J Pain.
2023 Apr;36(2):163-172. 10.3344/kjp.22372.
Calcium/calmodulin-dependent protein kinase II is involved in the transmission and regulation of nociception in naïve and morphine-tolerant rat nucleus accumbens
- Affiliations
-
- 1Department of Physiology, Faculty of Basic Medical Science, Kunming Medical University, Kunming, Yunnan, China
- 2Second Department of Neurosurgery, The First Affiliated Hospital, Kunming Medical University, Kunming, Yunnan, China
- 3Department of Cerebrovascular Surgery, Xinyu People’s Hospital, Xinyu, Jiangxi, China
- 4Department of Oncology, The Second Affiliated Hospital, Kunming Medical University, Kunming, Yunnan, China
- KMID: 2541025
- DOI: http://doi.org/10.3344/kjp.22372
Abstract
- Background
Synaptic plasticity contributes to nociceptive signal transmission and modulation, with calcium/ calmodulin-dependent protein kinase II (CaMK II) playing a fundamental role in neural plasticity. This research was conducted to investigate the role of CaMK II in the transmission and regulation of nociceptive information within the nucleus accumbens (NAc) of naïve and morphine-tolerant rats.
Methods
Randall Selitto and hot-plate tests were utilized to measure the hindpaw withdrawal latencies (HWLs) in response to noxious mechanical and thermal stimuli. To induce chronic morphine tolerance, rats received intraperitoneal morphine injection twice per day for seven days. CaMK II expression and activity were assessed using western blotting.
Results
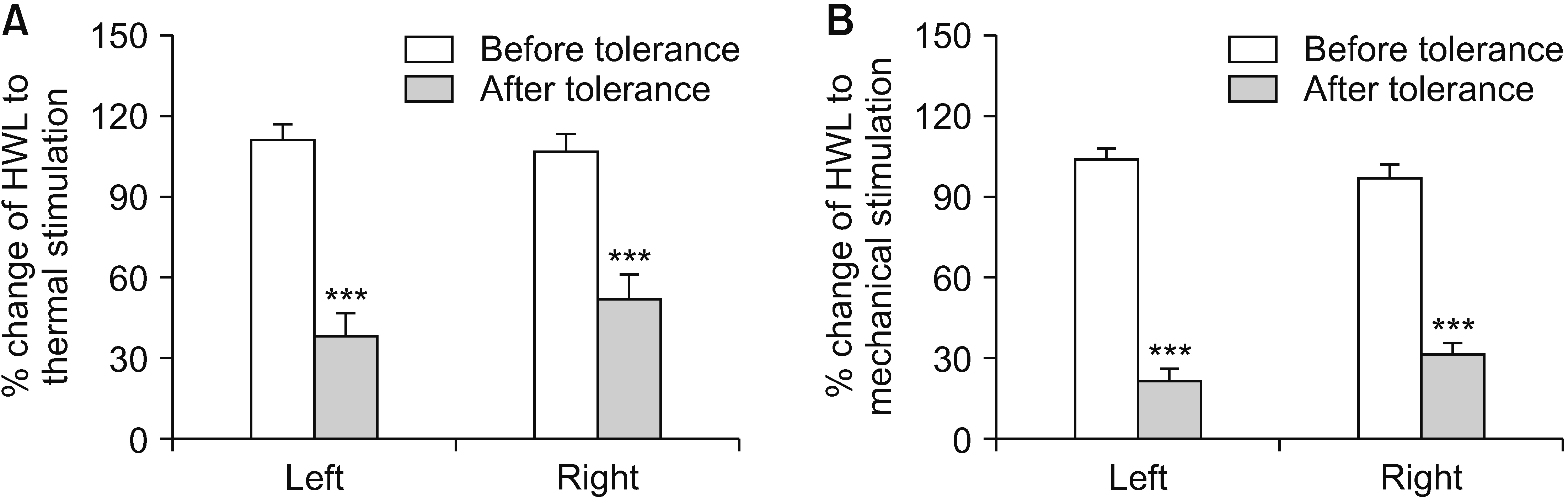
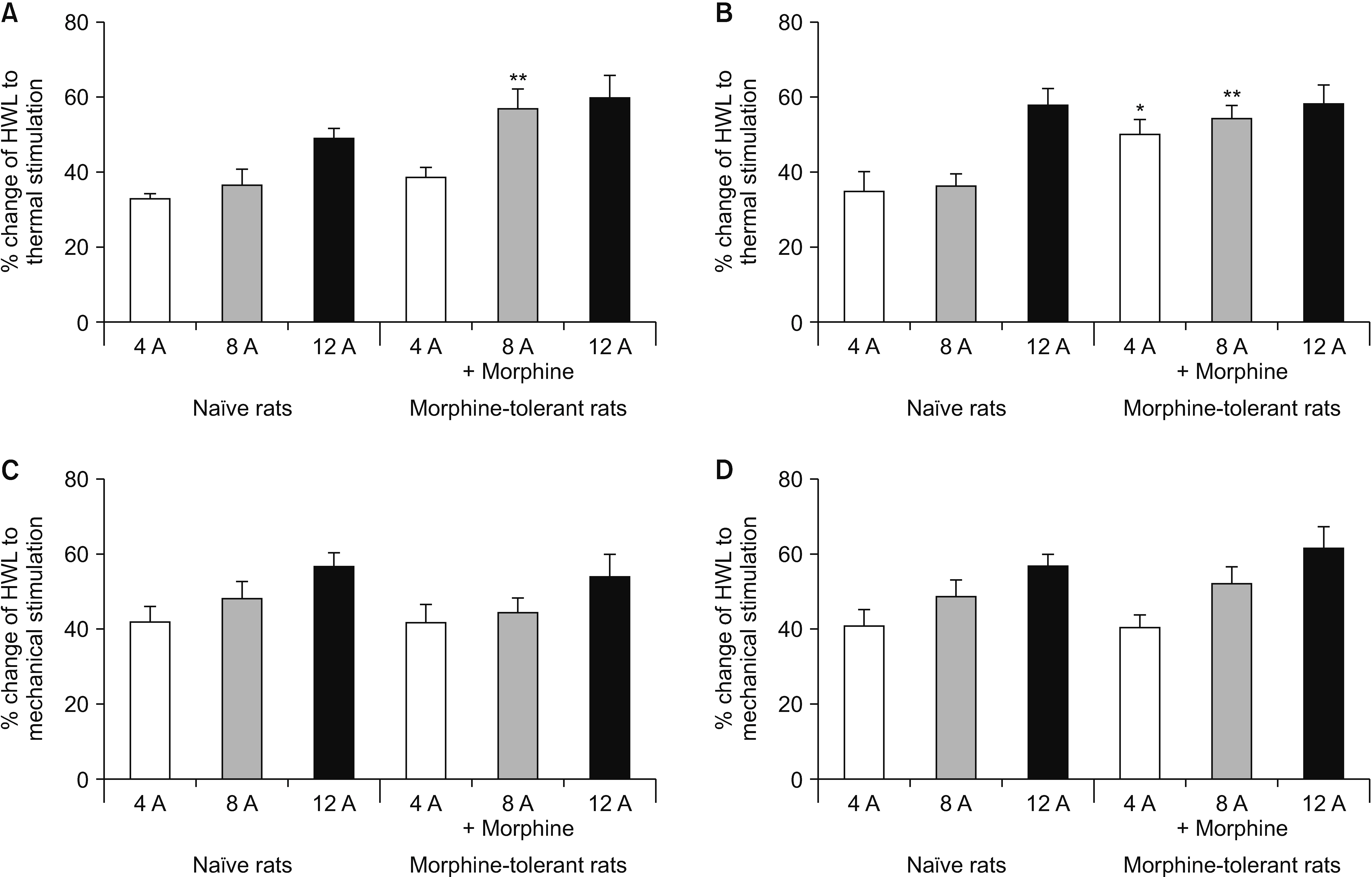
Intra-NAc microinjection of autocamtide-2-related inhibitory peptide (AIP) induced an increase in HWLs in naïve rats in response to noxious thermal and mechanical stimuli. Moreover, the expression of the phosphorylated CaMK II (p-CaMK II) was significantly decreased as determined by western blotting. Chronic intraperitoneal injection of morphine resulted in significant morphine tolerance in rats on Day 7, and an increase of p-CaMK II expression in NAc in morphine-tolerant rats was observed. Furthermore, intra-NAc administration of AIP elicited significant antinociceptive responses in morphine-tolerant rats. In addition, compared with naïve rats, AIP induced stronger thermal antinociceptive effects of the same dose in rats exhibiting morphine tolerance.
Conclusions
This study shows that CaMK II in the NAc is involved in the transmission and regulation of nociception in naïve and morphine-tolerant rats.
Keyword
Figure
Reference
-
1. Harris HN, Peng YB. 2020; Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regen Res. 15:597–605. DOI: 10.4103/1673-5374.266909. PMID: 31638081. PMCID: PMC6975138. PMID: 5ff4779640c543e98385c7128c875f7d.2. Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Luo F, et al. 2017; Cellular and molecular mechanisms of calcium/calmodulin-dependent protein kinase II in chronic pain. J Pharmacol Exp Ther. 363:176–83. DOI: 10.1124/jpet.117.243048. PMID: 28855373.3. Kim MJ, Kim JY, Lim YH, Hong SJ, Jeong JH, Choi HR, et al. 2022; Actual situation and prescribing patterns of opioids by pain physicians in South Korea. Korean J Pain. 35:475–87. DOI: 10.3344/kjp.2022.35.4.475. PMID: 36175347. PMCID: PMC9530690.4. Mackey S, Kao MC. 2019; Managing twin crises in chronic pain and prescription opioids. BMJ. 364:l917. DOI: 10.1136/bmj.l917. PMID: 30842099.5. Wang ZJ, Wang LX. 2006; Phosphorylation: a molecular switch in opioid tolerance. Life Sci. 79:1681–91. DOI: 10.1016/j.lfs.2006.05.023. PMID: 16831450.6. Zhang TJ, Qiu Y, Hua Z. 2019; The emerging perspective of morphine tolerance: microRNAs. Pain Res Manag. 2019:9432965. DOI: 10.1155/2019/9432965. PMID: 31182985. PMCID: PMC6515020. PMID: f2bde55923c7418caf5a7838791ce265.7. Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. 2016; Gliogenic LTP spreads widely in nociceptive pathways. Science. 354:1144–8. DOI: 10.1126/science.aah5715. PMID: 27934764. PMCID: PMC6145441.8. Christie MJ. 2008; Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 154:384–96. DOI: 10.1038/bjp.2008.100. PMID: 18414400. PMCID: PMC2442443.9. Fukushima H, Maeda R, Suzuki R, Suzuki A, Nomoto M, Toyoda H, et al. 2008; Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J Neurosci. 28:9910–9. DOI: 10.1523/JNEUROSCI.2625-08.2008. PMID: 18829949. PMCID: PMC6671266.10. Yang C, Chen Y, Tang L, Wang ZJ. 2011; Haloperidol disrupts opioid-antinociceptive tolerance and physical dependence. J Pharmacol Exp Ther. 338:164–72. DOI: 10.1124/jpet.110.175539. PMID: 21436292. PMCID: PMC3126635.11. Hu X, Huang F, Szymusiak M, Liu Y, Wang ZJ. 2015; Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II α activity. J Pharmacol Exp Ther. 352:420–8. DOI: 10.1124/jpet.114.219303. PMID: 25515789. PMCID: PMC4352596.12. Tang L, Shukla PK, Wang LX, Wang ZJ. 2006; Reversal of morphine antinociceptive tolerance and dependence by the acute supraspinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther. 317:901–9. DOI: 10.1124/jpet.105.097733. PMID: 16505162.13. Tang L, Shukla PK, Wang ZJ. 2006; Trifluoperazine, an orally available clinically used drug, disrupts opioid antinociceptive tolerance. Neurosci Lett. 397:1–4. DOI: 10.1016/j.neulet.2005.11.050. PMID: 16380209.14. Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. 1999; Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol. 56:39–45. DOI: 10.1124/mol.56.1.39. PMID: 10385682.15. Zhang Y, Gao Y, Li CY, Dong W, Li MN, Liu YN, et al. 2019; Galanin plays a role in antinociception via binding to galanin receptors in the nucleus accumbens of rats with neuropathic pain. Neurosci Lett. 706:93–8. DOI: 10.1016/j.neulet.2019.05.016. PMID: 31085289.16. Xiong W, Yu LC. 2006; Involvement of endogenous cholecystokinin in tolerance to morphine antinociception in the nucleus accumbens of rats. Behav Brain Res. 173:116–21. DOI: 10.1016/j.bbr.2006.06.010. PMID: 16837074.17. Bian H, Yu LC. 2014; Intra-nucleus accumbens administration of the calcium/calmodulin-dependent protein kinase II inhibitor KN93 induced antinociception in rats with mononeuropathy. Neurosci Lett. 583:6–10. DOI: 10.1016/j.neulet.2014.09.007. PMID: 25218714.18. Bian H, Yu LC. 2015; Intra-nucleus accumbens administration of the calcium/calmodulin-dependent protein kinase II inhibitor AIP induced antinociception in rats with mononeuropathy. Neurosci Lett. 599:129–32. DOI: 10.1016/j.neulet.2015.05.048. PMID: 26022629.19. Dong Y, Li CY, Zhang XM, Liu YN, Yang S, Li MN, et al. 2021; The activation of galanin receptor 2 plays an antinociceptive effect in nucleus accumbens of rats with neuropathic pain. J Physiol Sci. 71:6. DOI: 10.1186/s12576-021-00790-5. PMID: 33546583.20. Hou KS, Wang LL, Wang HB, Fu FH, Yu LC. 2020; Role of calcitonin gene-related peptide in nociceptive modulationin anterior cingulate cortex of naïve rats and rats with inflammatory pain. Front Pharmacol. 11:928. DOI: 10.3389/fphar.2020.00928. PMID: 32670060. PMCID: PMC7332858. PMID: db61c69bac7741b58213e2568441593e.21. Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates. 4th ed. Academic Press;Sydney: DOI: 10.1016/c2009-0-63235-9.22. Volkow ND, McLellan AT. 2016; Opioid abuse in chronic pain--misconceptions and mitigation strategies. N Engl J Med. 374:1253–63. DOI: 10.1056/NEJMra1507771. PMID: 27028915.23. Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, et al. 2017; Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med. 23:164–73. DOI: 10.1038/nm.4262. PMID: 28092666. PMCID: PMC5296291.24. Calcaterra S, Glanz J, Binswanger IA. 2013; National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths:. 1999-2009. Drug Alcohol Depend. 131:263–70. DOI: 10.1016/j.drugalcdep.2012.11.018. PMID: 23294765. PMCID: PMC3935414.25. Wang ZJ, Tang L, Xin L. 2003; Reversal of morphine antinociceptive tolerance by acute spinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. Eur J Pharmacol. 465:199–200. DOI: 10.1016/S0014-2999(03)01484-5. PMID: 12650850.26. Liang D, Li X, Clark JD. 2004; Increased expression of Ca2+/calmodulin-dependent protein kinase II alpha during chronic morphine exposure. Neuroscience. 123:769–75. DOI: 10.1016/j.neuroscience.2003.10.007. PMID: 14706789.27. Wang Z, Chabot JG, Quirion R. 2011; On the possible role of ERK, p38 and CaMKII in the regulation of CGRP expression in morphine-tolerant rats. Mol Pain. 7:68. DOI: 10.1186/1744-8069-7-68. PMID: 21933441. PMCID: PMC3190348. PMID: dae7c5fdcb484d66a4db0b5b8bac48dd.28. Wang Z, Ma W, Chabot JG, Quirion R. 2010; Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFκB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain. 151:194–205. DOI: 10.1016/j.pain.2010.07.006. PMID: 20691540.29. Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, et al. 1995; The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci. 15(3 Pt 2):2396–406. DOI: 10.1523/JNEUROSCI.15-03-02396.1995. PMID: 7891175. PMCID: PMC6578163.30. Koch T, Kroslak T, Mayer P, Raulf E, Höllt V. 1997; Site mutation in the rat mu-opioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J Neurochem. 69:1767–70. DOI: 10.1046/j.1471-4159.1997.69041767.x. PMID: 9326307.31. Brüggemann I, Schulz S, Wiborny D, Höllt V. 2000; Colocalization of the mu-opioid receptor and calcium/calmodulin-dependent kinase II in distinct pain-processing brain regions. Brain Res Mol Brain Res. 85:239–50. DOI: 10.1016/S0169-328X(00)00265-5. PMID: 11146127.32. Zhu QM, Wu LX, Zhang B, Dong YP, Sun L. 2021; Donepezil prevents morphine tolerance by regulating N-methyl-d-aspartate receptor, protein kinase C and CaM-dependent kinase II expression in rats. Pharmacol Biochem Behav. 206:173209. DOI: 10.1016/j.pbb.2021.173209. PMID: 34058253.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Calcium/calmodulin-dependent protein kinase II is involved in the transmission and regulation of nociception in naïve and morphine-tolerant rat nucleus accumbens
- Role of Calmodulin in the Generation of Reactive Oxygen Species and Apoptosis Induced by Tamoxifen in HepG2 Human Hepatoma Cells
- A Memory Molecule, Ca2+/Calmodulin-Dependent Protein Kinase II and Redox Stress; Key Factors for Arrhythmias in a Diseased Heart
- Protective effects of kaempferol against cardiac sinus node dysfunction via CaMKII deoxidization
- Regulation of Protein Kinase in KCl-induced Contraction of Cat Gastric Smooth Muscle