Anat Cell Biol.
2023 Mar;56(1):69-85. 10.5115/acb.22.120.
Boophone disticha attenuates five day repeated forced swim-induced stress and adult hippocampal neurogenesis impairment in male Balb/c mice
- Affiliations
-
- 1School of Anatomical Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 2Department of Anatomy, School of Medicine, Sefako Magkatho Health Sciences University, Pretoria, South Africa
- 3Department of Human Anatomy and Physiology, Faculty of Health Sciences, University of Johannesburg, Johannesburg, South Africa
- KMID: 2540987
- DOI: http://doi.org/10.5115/acb.22.120
Abstract
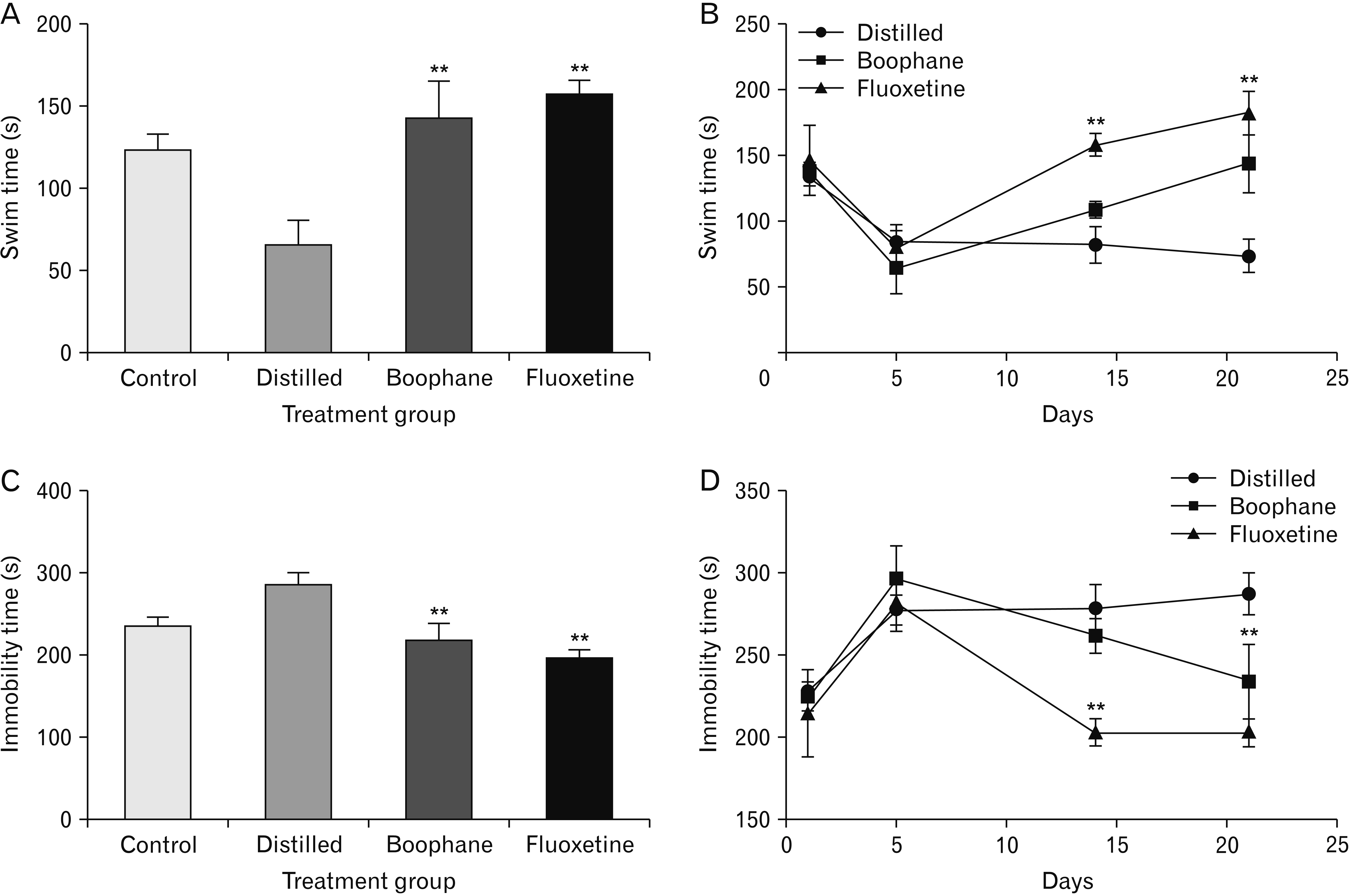
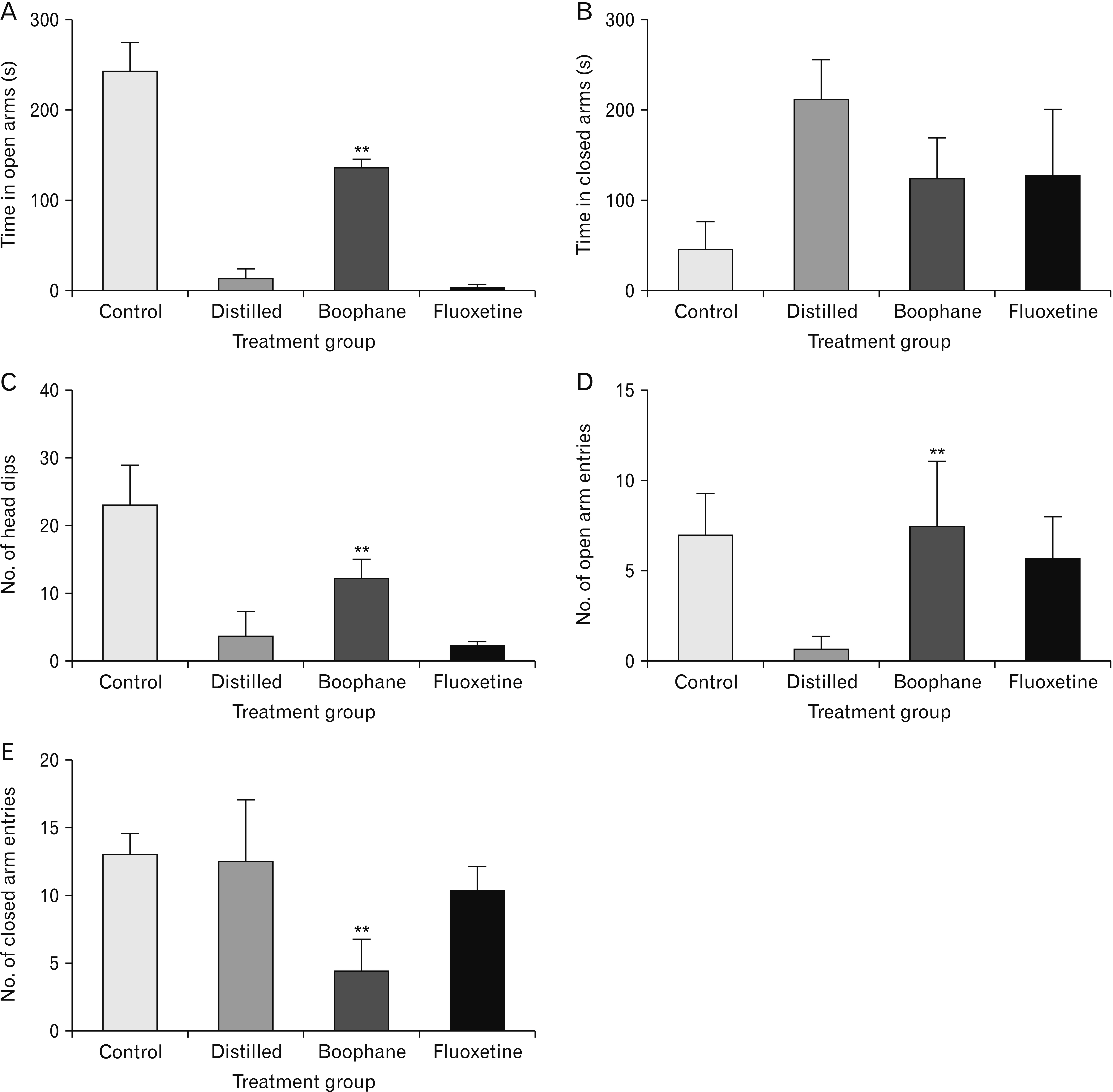
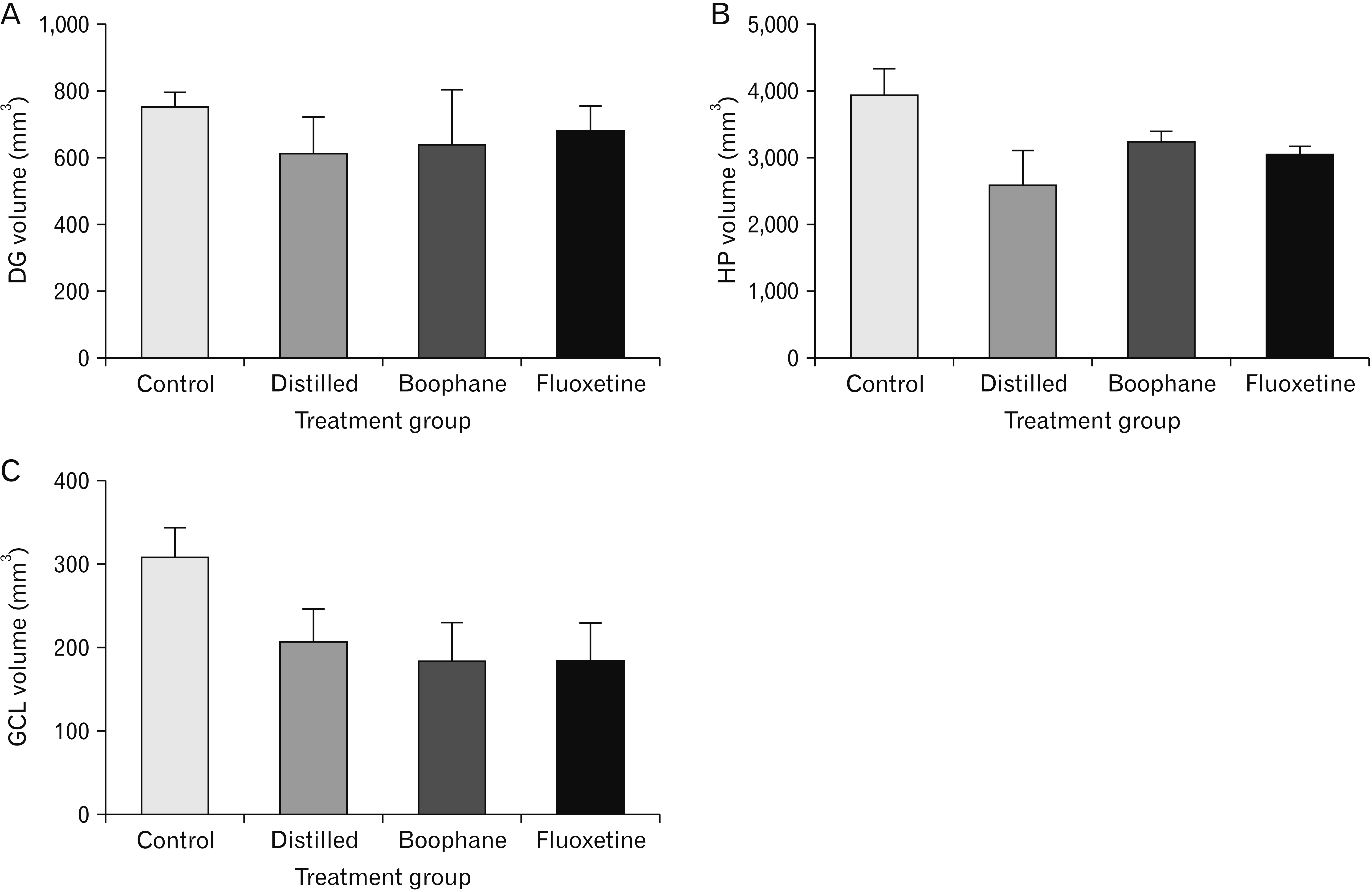
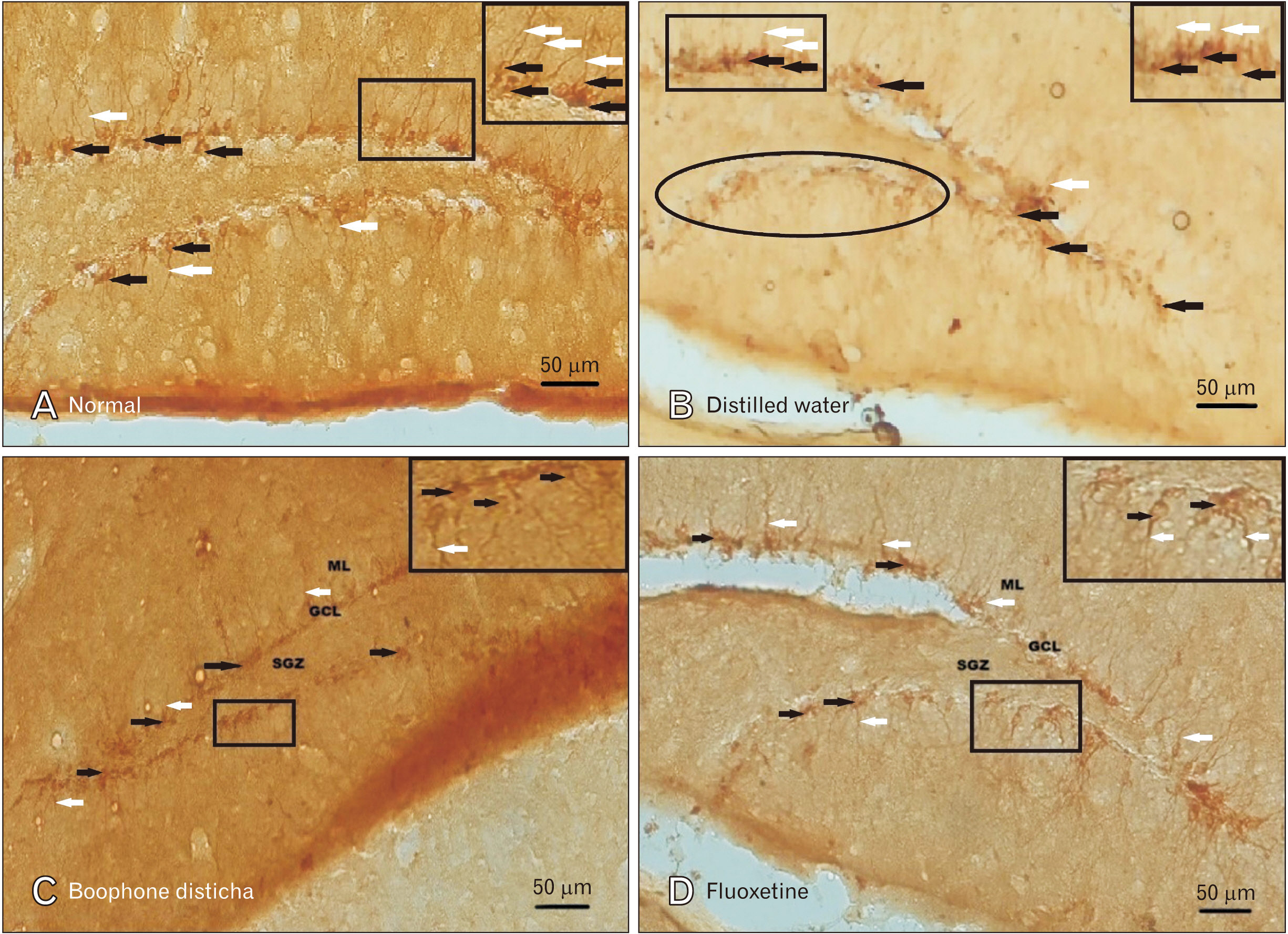
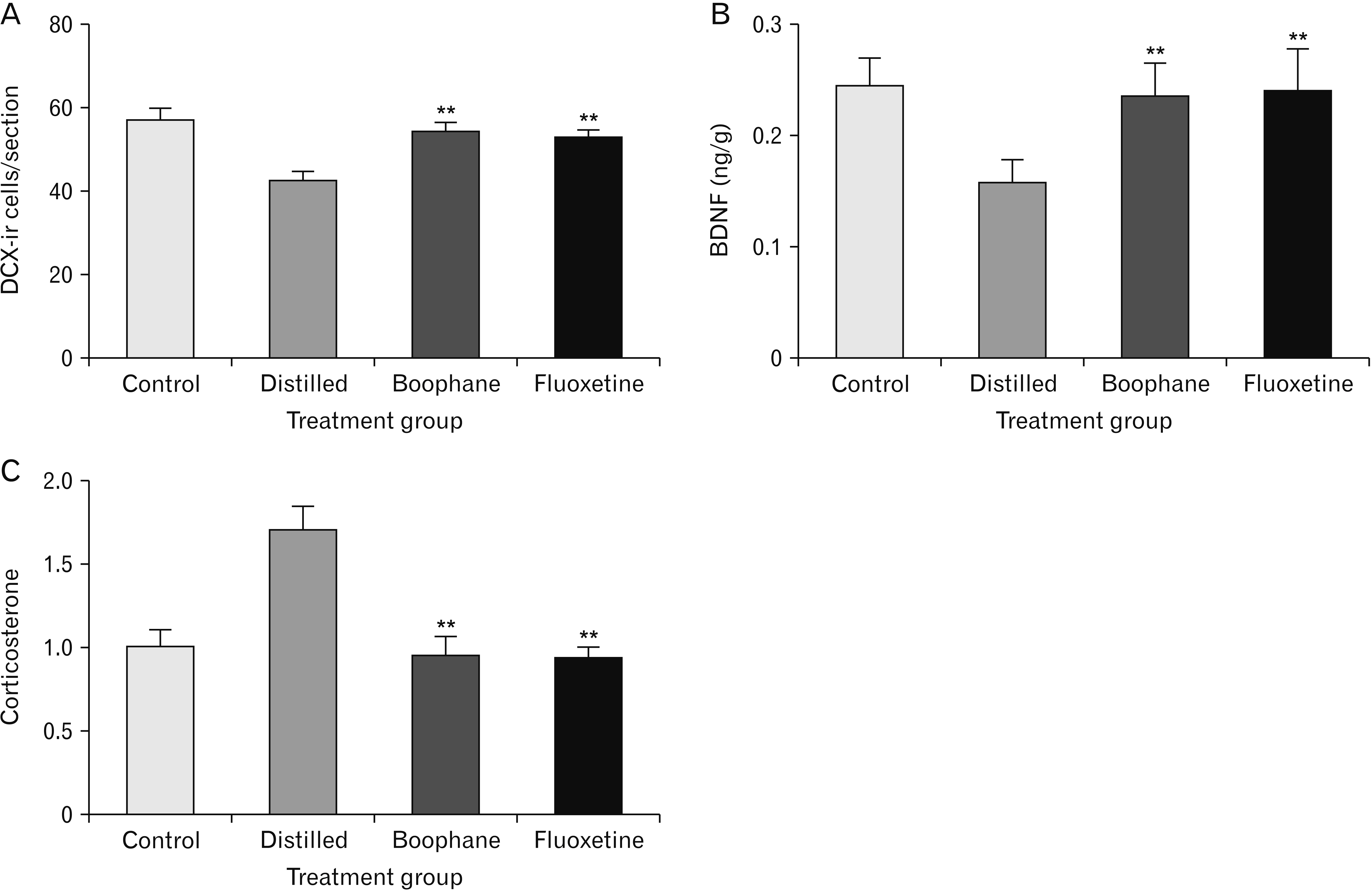
- Depression is one of the most common neuropsychiatric disorders and is associated with dysfunction of the neuroendocrine system and alterations in specific brain proteins. Boophone disticha (BD) is an indigenous psychoactive bulb that belongs to the Amaryllidacae family, which is widely used in Southern Africa to treat depression, with scientific evidence of potent antidepressant-like effects. The present study examined the antidepressant effects of BD and its mechanisms of action by measuring some behavioural parameters in the elevated plus maze, brain content of corticosterone, brain derived neurotropic factor (BDNF), and neuroblast differentiation in the hippocampus of Balb/c mice exposed to the five day repeated forced swim stress (5d-RFSS). Male Balb/c mice were subjected to the 5d-RFSS protocol to induce depressive-like behaviour (decreased swimming, increased floating, decreased open arm entry, decreased time spent in the open arms and decreased head dips in the elevated plus maze test) and treated with distilled water, fluoxetine and BD. BD treatment (10 mg/kg/p.o for 3 weeks) significantly attenuated the 5d-RFSS-induced behavioural abnormalities and the elevated serum corticosterone levels observed in stressed mice. Additionally, 5d-RFSS exposure significantly decreased the number of neuroblasts in the hippocampus and BDNF levels in the brain of Balb/c mice, while fluoxetine and BD treatment attenuated these changes. The antidepressant effects of BD were comparable to those of fluoxetine, but unlike fluoxetine, BD did not show any anxiogenic effects, suggesting better pharmacological functions. In conclusion, our study shows that BD exerted antidepressant-like effects in 5d-RFSS mice, mediated in part by normalizing brain corticosterone and BDNF levels.
Figure
Reference
-
References
1. Vigo D, Thornicroft G, Atun R. 2016; Estimating the true global burden of mental illness. Lancet Psychiatry. 3:171–8. DOI: 10.1016/S2215-0366(15)00505-2. PMID: 26851330.
Article2. Zayka TO, Evdokimov DV, Sidorova YV, Abramets II, Nalotov SV. 2015; Antidepressant-like effects of substances with cerebroprotective activity. Biol Markers Guided Ther. 2:79–88. DOI: 10.12988/bmgt.2015.5117.
Article3. Anacker C. 2014; Fresh approaches to antidepressant drug discovery. Expert Opin Drug Discov. 9:407–21. DOI: 10.1517/17460441.2014.892071. PMID: 24588262.
Article4. Hornung OP, Heim CM. 2014; Gene-environment interactions and intermediate phenotypes: early trauma and depression. Front Endocrinol (Lausanne). 5:14. DOI: 10.3389/fendo.2014.00014. PMID: 24596569. PMCID: PMC3925849.
Article5. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. 2003; The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 289:3095–105. DOI: 10.1001/jama.289.23.3095. PMID: 12813115.6. Kharamin S, Razmeh S, Nabovvati M, Moradian K, Rahimi S, Orooji M, Taghavian L, Mand Maher MK. 2019; Evaluation of antidepressant-like effect Lavandulifolia stachys in the forced swimming test in comparison with imipramine and fluoxetine. Int J Plant Biol. 10:7458. DOI: 10.4081/pb.2019.7458.
Article7. Panwar R, Sivakumar M, Menon V, Vairappan B. 2020; Changes in the levels of comet parameters before and after fluoxetine therapy in major depression patients. Anat Cell Biol. 53:194–200. DOI: 10.5115/acb.19.217. PMID: 32647087. PMCID: PMC7343562.
Article8. Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. 2001; Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 4:399–408. DOI: 10.1017/S1461145701002632. PMID: 11806866.
Article9. Nutt DJ. 2005; Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 10:49–56. DOI: 10.1017/S1092852900009901. PMID: 15618947.
Article10. Birkett MA, Shinday NM, Kessler EJ, Meyer JS, Ritchie S, Rowlett JK. 2011; Acute anxiogenic-like effects of selective serotonin reuptake inhibitors are attenuated by the benzodiazepine diazepam in BALB/c mice. Pharmacol Biochem Behav. 98:544–51. DOI: 10.1016/j.pbb.2011.03.006. PMID: 21397628. PMCID: PMC3085089.
Article11. Ramaekers JG. 2003; Antidepressants and driver impairment: empirical evidence from a standard on-the-road test. J Clin Psychiatry. 64:20–9. DOI: 10.4088/JCP.v64n0106. PMID: 12590619.12. Sandager M, Nielsen ND, Stafford GI, van Staden J, Jäger AK. 2005; Alkaloids from Boophane disticha with affinity to the serotonin transporter in rat brain. J Ethnopharmacol. 98:367–70. DOI: 10.1016/j.jep.2005.01.037. PMID: 15814274.
Article13. Gadaga LL, Tagwireyi D, Dzangare J, Nhachi CF. 2011; Acute oral toxicity and neurobehavioural toxicological effects of hydroethanolic extract of Boophone disticha in rats. Hum Exp Toxicol. 30:972–80. DOI: 10.1177/0960327110384524. PMID: 20889580.
Article14. Pote W, Tagwireyi D, Chinyanga HM, Musara C, Pfukenyi DM, Nkomozepi P, Gadaga LL, Nyandoro G, Chifamba J. 2014; Long-term cardiovascular autonomic responses to aqueous ethanolic extract of Boophone disticha bulb in early maternally separated BALB/c mice. S Afr J Bot. 94:33–9. DOI: 10.1016/j.sajb.2014.04.012.
Article15. Pote W, Musarira S, Chuma D, Gadaga LL, Mwandiringana E, Tagwireyi D. 2018; Effects of a hydroethanolic extract of Boophone disticha bulb on anxiety-related behaviour in naive BALB/c mice. J Ethnopharmacol. 214:218–24. DOI: 10.1016/j.jep.2017.12.001. PMID: 29223391.
Article16. Pedersen ME, Szewczyk B, Stachowicz K, Wieronska J, Andersen J, Stafford GI, van Staden J, Pilc A, Jäger AK. 2008; Effects of South African traditional medicine in animal models for depression. J Ethnopharmacol. 119:542–8. DOI: 10.1016/j.jep.2008.08.030. PMID: 18809486.
Article17. Cheesman L, Nair JJ, van Staden J. 2012; Antibacterial activity of crinane alkaloids from Boophone disticha (Amaryllidaceae). J Ethnopharmacol. 140:405–8. DOI: 10.1016/j.jep.2012.01.037. PMID: 22322252.
Article18. Nkomozepi P, Mazengenya P, Ihunwo AO. 2019; Age-related changes in Ki-67 and DCX expression in the BALB/c mouse (Mus Musculus) brain. Int J Dev Neurosci. 72:36–47. DOI: 10.1016/j.ijdevneu.2018.11.005. PMID: 30472241.
Article19. Song NN, Huang Y, Yu X, Lang B, Ding YQ, Zhang L. 2017; Divergent roles of central serotonin in adult hippocampal neurogenesis. Front Cell Neurosci. 11:185. DOI: 10.3389/fncel.2017.00185. PMID: 28713247. PMCID: PMC5492328.
Article20. Malykhin NV, Coupland NJ. 2015; Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 309:200–13. DOI: 10.1016/j.neuroscience.2015.04.047. PMID: 25934030.
Article21. Mul JD, Zheng J, Goodyear LJ. 2016; Validity assessment of 5 day repeated forced-swim stress to model human depression in young-adult C57BL/6J and BALB/cJ mice. eNeuro. 3:ENEURO.0201-16.2016. DOI: 10.1523/ENEURO.0201-16.2016. PMID: 28058270. PMCID: PMC5197406.
Article22. Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A, Normann C, Biber K, van Calker D. 2015; Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of homer1a. Neuron. 87:549–62. DOI: 10.1016/j.neuron.2015.07.010. PMID: 26247862. PMCID: PMC4803038.
Article23. Hodes GE, Hill-Smith TE, Lucki I. 2010; Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett. 484:12–6. DOI: 10.1016/j.neulet.2010.07.084. PMID: 20692322. PMCID: PMC4623584.
Article24. Pawluski JL, van Donkelaar E, Abrams Z, Houbart V, Fillet M, Steinbusch HW, Charlier TD. 2014; Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats. Neural Plast. 2014:123026. DOI: 10.1155/2014/123026. PMID: 24757568. PMCID: PMC3976918.
Article25. Porsolt RD, Bertin A, Jalfre M. 1977; Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 229:327–36. PMID: 596982.26. Kara NZ, Stukalin Y, Einat H. 2018; Revisiting the validity of the mouse forced swim test: systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci Biobehav Rev. 84:1–11. DOI: 10.1016/j.neubiorev.2017.11.003. PMID: 29128579.
Article27. PETA. c2020. Victories! PETA is ending near-drowning experiments on animals [Internet]. People for the Ethical Treatment of Animals;Norfolk, VA: Available from: https://www.peta.org/features/peta-ends-near-drowning-tests-small-animals. cited 2022 Jul 18.28. LASA. c2020. The forced swim test [Internet]. LASA;Hull: Available from: https://www.lasa.co.uk/the-forced-swim-test. cited 2022 Jul 22.29. Sewell F, Waterson I, Jones D, Tricklebank MD, Ragan I. 2021; Preclinical screening for antidepressant activity - shifting focus away from the Forced Swim Test to the use of translational biomarkers. Regul Toxicol Pharmacol. 125:105002. DOI: 10.1016/j.yrtph.2021.105002. PMID: 34245825.
Article30. Nalloor R, Bunting K, Vazdarjanova A. 2011; Predicting impaired extinction of traumatic memory and elevated startle. PLoS One. 6:e19760. DOI: 10.1371/journal.pone.0019760. PMID: 21611173. PMCID: PMC3097191.
Article31. Walf AA, Frye CA. 2007; The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2:322–8. DOI: 10.1038/nprot.2007.44. PMID: 17406592. PMCID: PMC3623971.
Article32. Schneider CA, Rasband WS, Eliceiri KW. 2012; NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–5. DOI: 10.1038/nmeth.2089. PMID: 22930834. PMCID: PMC5554542.
Article33. Zhang L, Li J, Lin A. 2021; Assessment of neurodegeneration and neuronal loss in aged 5XFAD mice. STAR Protoc. 2:100915. DOI: 10.1016/j.xpro.2021.100915. PMID: 34755118. PMCID: PMC8556527.
Article34. Neergaard JS, Andersen J, Pedersen ME, Stafford GI, Staden JV, Jäger AK. 2009; Alkaloids from Boophone disticha with affinity to the serotonin transporter. S Afr J Bot. 75:371–4. DOI: 10.1016/j.sajb.2009.02.173.
Article35. Dulawa SC, Holick KA, Gundersen B, Hen R. 2004; Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 29:1321–30. DOI: 10.1038/sj.npp.1300433. PMID: 15085085.
Article36. Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-de-Oliveira C. 2011; Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J Neurosci Methods. 195:200–5. DOI: 10.1016/j.jneumeth.2010.12.015. PMID: 21167866.
Article37. Detke MJ, Rickels M, Lucki I. 1995; Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl). 121:66–72. DOI: 10.1007/BF02245592. PMID: 8539342.
Article38. Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. 1999; Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl). 147:162–7. DOI: 10.1007/s002130051156. PMID: 10591883.
Article39. Handley SL, McBlane JW. 1993; An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 29:129–38. DOI: 10.1016/1056-8719(93)90063-K. PMID: 8103377.
Article40. Rocher C, Spedding M, Munoz C, Jay TM. 2004; Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 14:224–9. Erratum in: Cereb Cortex 2004;14:352. DOI: 10.1093/cercor/bhg122. PMID: 14704220.
Article41. Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ. 2009; Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 20:1554–8. DOI: 10.1097/WNR.0b013e328332bb09. PMID: 19858767. PMCID: PMC2783199.
Article42. Malhi GS, Das P, Outhred T, Dobson-Stone C, Irwin L, Gessler D, Bryant R, Mannie Z. 2019; Effect of stress gene-by-environment interactions on hippocampal volumes and cortisol secretion in adolescent girls. Aust N Z J Psychiatry. 53:316–25. DOI: 10.1177/0004867419827649. PMID: 30754992.
Article43. Moica T, Gligor A, Moica S. 2016; The relationship between cortisol and the hippocampal volume in depressed patients - a MRI pilot study. Proced Technol. 22:1106–12. DOI: 10.1016/j.protcy.2016.01.156.
Article44. Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O'Mara SM. 2016; Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 6:29127. DOI: 10.1038/srep29127. PMID: 27374165. PMCID: PMC4931588.
Article45. Zimmerman ME, Ezzati A, Katz MJ, Lipton ML, Brickman AM, Sliwinski MJ, Lipton RB. 2016; Perceived stress is differentially related to hippocampal subfield volumes among older adults. PLoS One. 11:e0154530. DOI: 10.1371/journal.pone.0154530. PMID: 27144832. PMCID: PMC4856349.
Article46. Pham K, Nacher J, Hof PR, McEwen BS. 2003; Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 17:879–86. DOI: 10.1046/j.1460-9568.2003.02513.x. PMID: 12603278.
Article47. Mirescu C, Gould E. 2006; Stress and adult neurogenesis. Hippocampus. 16:233–8. DOI: 10.1002/hipo.20155. PMID: 16411244.
Article48. Marečková K, Mareček R, Bencurova P, Klánová J, Dušek L, Brázdil M. 2018; Perinatal stress and human hippocampal volume: findings from typically developing young adults. Sci Rep. 8:4696. DOI: 10.1038/s41598-018-23046-6. PMID: 29549289. PMCID: PMC5856850.
Article49. Maguire EA, Woollett K, Spiers HJ. 2006; London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 16:1091–101. DOI: 10.1002/hipo.20233. PMID: 17024677.
Article50. Leuner B, Gould E. 2010; Structural plasticity and hippocampal function. Annu Rev Psychol. 61:111–40. C1–3. DOI: 10.1146/annurev.psych.093008.100359. PMID: 19575621. PMCID: PMC3012424.
Article51. Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, Suzuki H. 2010; Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci U S A. 107:8434–9. DOI: 10.1073/pnas.0912690107. PMID: 20404165. PMCID: PMC2889553.
Article52. Ohira K, Miyakawa T. 2011; Chronic treatment with fluoxetine for more than 6 weeks decreases neurogenesis in the subventricular zone of adult mice. Mol Brain. 4:10. DOI: 10.1186/1756-6606-4-10. PMID: 21385396. PMCID: PMC3060120.
Article53. Ohira K, Hagihara H, Miwa M, Nakamura K, Miyakawa T. 2019; Fluoxetine-induced dematuration of hippocampal neurons and adult cortical neurogenesis in the common marmoset. Mol Brain. 12:69. DOI: 10.1186/s13041-019-0489-5. PMID: 31383032. PMCID: PMC6683334. PMID: e232f729694340f5b197eb5d287f33cb.
Article54. Shuto T, Kuroiwa M, Sotogaku N, Kawahara Y, Oh YS, Jang JH, Shin CH, Ohnishi YN, Hanada Y, Miyakawa T, Kim Y, Greengard P, Nishi A. 2020; Obligatory roles of dopamine D1 receptors in the dentate gyrus in antidepressant actions of a selective serotonin reuptake inhibitor, fluoxetine. Mol Psychiatry. 25:1229–44. DOI: 10.1038/s41380-018-0316-x. PMID: 30531938. PMCID: PMC7244404.
Article55. Encinas JM, Vaahtokari A, Enikolopov G. 2006; Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 103:8233–8. DOI: 10.1073/pnas.0601992103. PMID: 16702546. PMCID: PMC1461404.
Article56. Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. 2008; Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 28:1374–84. DOI: 10.1523/JNEUROSCI.3632-07.2008. PMID: 18256257. PMCID: PMC6671574.
Article57. Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. 2013; Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 38:1068–77. DOI: 10.1038/npp.2013.5. PMID: 23303074. PMCID: PMC3629406.
Article58. Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, Madroñal N, Donaldson ZR, Drew LJ, Dranovsky A, Gross CT, Tanaka KF, Hen R. 2015; 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 18:1606–16. DOI: 10.1038/nn.4116. PMID: 26389840. PMCID: PMC4624493.
Article59. Turcotte-Cardin V, Vahid-Ansari F, Luckhart C, Daigle M, Geddes SD, Tanaka KF, Hen R, James J, Merali Z, Béïque JC, Albert PR. 2019; Loss of adult 5-HT1A autoreceptors results in a paradoxical anxiogenic response to antidepressant treatment. J Neurosci. 39:1334–46. DOI: 10.1523/JNEUROSCI.0352-18.2018. PMID: 30552180. PMCID: PMC6381250.
Article60. Klempin F, Babu H, De Pietri Tonelli D, Alarcon E, Fabel K, Kempermann G. 2010; Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci. 3:14. DOI: 10.3389/fnmol.2010.00014. PMID: 20721314. PMCID: PMC2922940.
Article61. Arnold SA, Hagg T. 2012; Serotonin 1A receptor agonist increases species- and region-selective adult CNS proliferation, but not through CNTF. Neuropharmacology. 63:1238–47. DOI: 10.1016/j.neuropharm.2012.07.047. PMID: 22884499. PMCID: PMC3438376.
Article62. Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Römer S, Gardier AM, Hen R. 2014; Rapid anxiolytic effects of a 5-HT₄ receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology. 39:1366–78. DOI: 10.1038/npp.2013.332. PMID: 24287720. PMCID: PMC3988540.63. Browne CA, Hanke J, Rose C, Walsh I, Foley T, Clarke G, Schwegler H, Cryan JF, Yilmazer-Hanke D. 2014; Effect of acute swim stress on plasma corticosterone and brain monoamine levels in bidirectionally selected DxH recombinant inbred mouse strains differing in fear recall and extinction. Stress. 17:471–83. DOI: 10.3109/10253890.2014.954104. PMID: 25117886. PMCID: PMC4527314.
Article64. Habr SF, Macrini DJ, Florio JC, Bernardi MM. 2014; Repeated forced swim stress has additive effects in anxiety behavior and in cathecolamine levels of adult rats exposed to deltamethrin. Neurotoxicol Teratol. 46:57–61. DOI: 10.1016/j.ntt.2014.10.001. PMID: 25444720.
Article65. Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH. 2015; Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 10:e0117503. DOI: 10.1371/journal.pone.0117503. PMID: 25699675. PMCID: PMC4336318. PMID: d26e6ee3abcc48ee981c0795b090ada3.
Article66. Wasinski F, Estrela GR, Arakaki AM, Bader M, Alenina N, Klempin F, Araújo RC. 2016; Maternal forced swimming reduces cell proliferation in the postnatal dentate gyrus of mouse offspring. Front Neurosci. 10:402. DOI: 10.3389/fnins.2016.00402. PMID: 27621701. PMCID: PMC5002407.
Article67. Emiliano S, Ana Cecilia L, Karen B, Guillermo H, Nancy R. 2019; Effect of prenatal stress and forced swimming acute stress on adult rat's skeletal muscle and liver MDA levels. MOJ Anat Physiol. 6:266–31. DOI: 10.15406/mojap.2019.06.00277.68. Safari MA, Koushkie Jahromi M, Rezaei R, Aligholi H, Brand S. 2020; The effect of swimming on anxiety-like behaviors and corticosterone in stressed and unstressed rats. Int J Environ Res Public Health. 17:6675. DOI: 10.3390/ijerph17186675. PMID: 32937768. PMCID: PMC7558513. PMID: 7a7491c33ea34dc4ad2bb64f82c78a0a.
Article69. Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M. 2007; HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 155:245–56. DOI: 10.1016/j.pscychresns.2006.11.002. PMID: 17587554.
Article70. Knorr U, Vinberg M, Gether U, Winkel P, Gluud C, Wetterslev J, Kessing LV. 2012; The effect of escitalopram versus placebo on perceived stress and salivary cortisol in healthy first-degree relatives of patients with depression-a randomised trial. Psychiatry Res. 200:354–60. DOI: 10.1016/j.psychres.2012.05.015. PMID: 22703642.
Article71. Hernandez ME, Mendieta D, Pérez-Tapia M, Bojalil R, Estrada-Garcia I, Estrada-Parra S, Pavón L. 2013; Effect of selective serotonin reuptake inhibitors and immunomodulator on cytokines levels: an alternative therapy for patients with major depressive disorder. Clin Dev Immunol. 2013:267871. DOI: 10.1155/2013/267871. PMID: 24348675. PMCID: PMC3855951.
Article72. Ruhé HG, Khoenkhoen SJ, Ottenhof KW, Koeter MW, Mocking RJ, Schene AH. 2015; Longitudinal effects of the SSRI paroxetine on salivary cortisol in Major Depressive Disorder. Psychoneuroendocrinology. 52:261–71. DOI: 10.1016/j.psyneuen.2014.10.024. PMID: 25544738.
Article73. Inder WJ, Prickett TC, Mulder RT, Donald RA, Joyce PR. 2001; Reduction in basal afternoon plasma ACTH during early treatment of depression with fluoxetine. Psychopharmacology (Berl). 156:73–8. DOI: 10.1007/s002130100737. PMID: 11465636.
Article74. Paile-Hyvärinen M, Wahlbeck K, Eriksson JG. 2003; Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: a single-blind randomised placebo controlled trial. BMC Fam Pract. 4:7. DOI: 10.1186/1471-2296-4-7. PMID: 12747810. PMCID: PMC165418.
Article75. Hinkelmann K, Moritz S, Botzenhardt J, Muhtz C, Wiedemann K, Kellner M, Otte C. 2012; Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: a longitudinal study. Psychoneuroendocrinology. 37:685–92. DOI: 10.1016/j.psyneuen.2011.08.012. PMID: 21944955.
Article76. Aydemir O, Deveci A, Taneli F. 2005; The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 29:261–5. DOI: 10.1016/j.pnpbp.2004.11.009. PMID: 15694233.
Article77. Castrén E, Rantamäki T. 2010; The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 70:289–97. DOI: 10.1002/dneu.20758. PMID: 20186711.
Article78. Nagahara AH, Tuszynski MH. 2011; Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 10:209–19. DOI: 10.1038/nrd3366. PMID: 21358740.
Article79. Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E. 2012; Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 17:1130–42. DOI: 10.1038/mp.2011.113. PMID: 21912391. PMCID: PMC3237836.
Article80. Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. 2012; Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 169:1194–202. DOI: 10.1176/appi.ajp.2012.12020248. PMID: 23128924. PMCID: PMC3638149.
Article81. Wu LM, Hu MH, Tong XH, Han H, Shen N, Jin RT, Wang W, Zhou GX, He GP, Liu YS. 2012; Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. PLoS One. 7:e52331. DOI: 10.1371/journal.pone.0052331. PMID: 23284991. PMCID: PMC3527516. PMID: 7615391004254dfba9d52ffa95c2ff6d.
Article82. Phillips C. 2017; Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017:7260130. DOI: 10.1155/2017/7260130. PMID: 28928987. PMCID: PMC5591905.
Article83. Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. 2007; Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 61:187–97. DOI: 10.1016/j.biopsych.2006.03.021. PMID: 16697351.
Article84. Lindholm JS, Castrén E. 2014; Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci. 8:143. DOI: 10.3389/fnbeh.2014.00143. PMID: 24817844. PMCID: PMC4012208.
Article85. Nibuya M, Morinobu S, Duman RS. 1995; Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 15:7539–47. DOI: 10.1523/JNEUROSCI.15-11-07539.1995. PMID: 7472505. PMCID: PMC6578063.
Article86. Nibuya M, Nestler EJ, Duman RS. 1996; Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 16:2365–72. DOI: 10.1523/JNEUROSCI.16-07-02365.1996. PMID: 8601816. PMCID: PMC6578518.
Article87. Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L. 2008; The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 320:385–8. DOI: 10.1126/science.1150516. PMID: 18420937.
Article88. Castrén E. 2014; Neurotrophins and psychiatric disorders. Handb Exp Pharmacol. 220:461–79. DOI: 10.1007/978-3-642-45106-5_17. PMID: 24668483.
Article89. Castrén E, Kojima M. 2017; Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 97(Pt B):119–26. DOI: 10.1016/j.nbd.2016.07.010. PMID: 27425886.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repeated restraint stress promotes hippocampal neuronal cell ciliogenesis and proliferation in mice
- Hippocampal Neurogenesis and Phenotypic Differentiation after Pilocarpine-Induced Seizures in Young Mice
- Comparison of Neurite Outgrowth Induced by Erythropoietin (EPO) and Carbamylated Erythropoietin (CEPO) in Hippocampal Neural Progenitor Cells
- Normal Adult Hippocampal Neurogenesis in SRG3-overexpressing Transgenic Mice
- Chronic Treatment with Combined Chemotherapeutic Agents Affects Hippocampal Micromorphometry and Function in Mice, Independently of Neuroinflammation