J Liver Cancer.
2023 Mar;23(1):213-218. 10.17998/jlc.2023.02.23.
A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab
- Affiliations
-
- 1Department of Internal Medicine, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea
- 2Department of Radiation Oncology, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea
- KMID: 2540834
- DOI: http://doi.org/10.17998/jlc.2023.02.23
Abstract
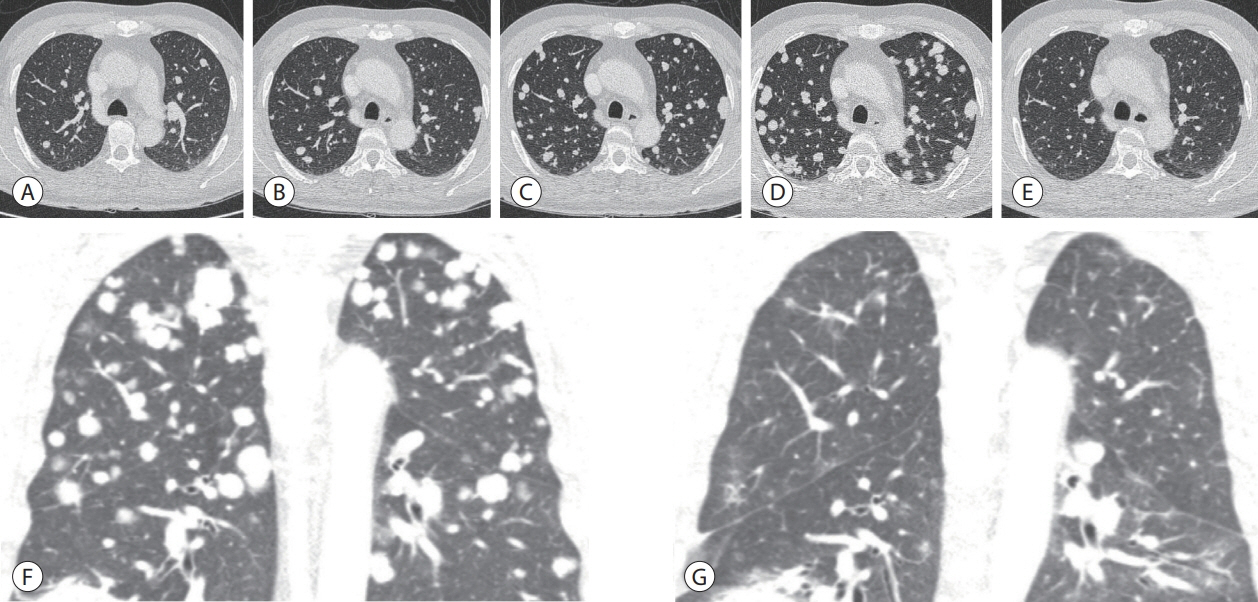
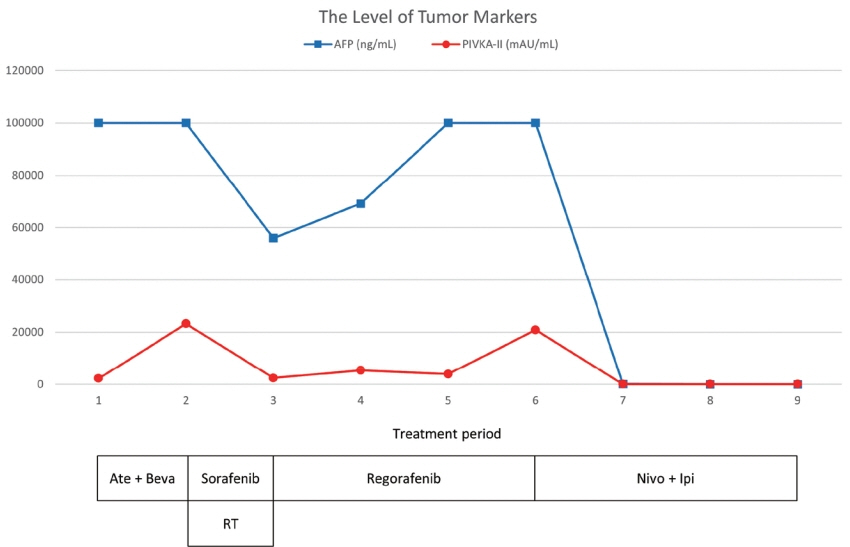
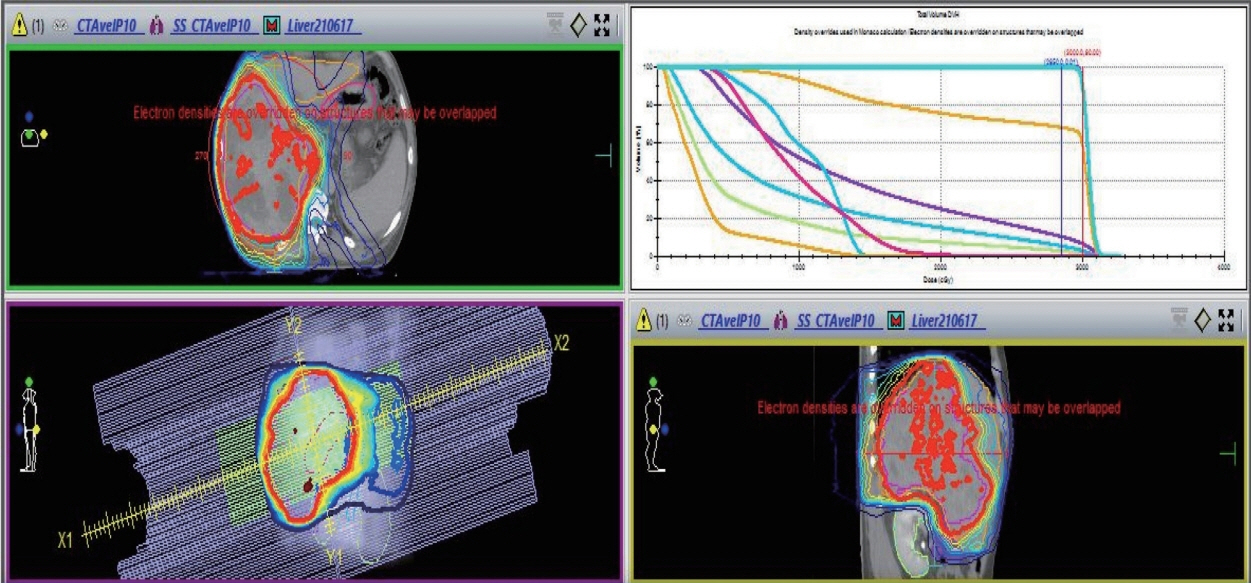
- Recently, the efficacy of immuno-oncologic agents for advanced hepatocellular carcinoma (HCC) has been proven in several trials. In particular, atezolizumab with bevacizumab (AteBeva), as a first-line therapy for advanced HCC, has shown tremendous advances in the IMBrave150 study. However, second or third-line therapy after treatment failure with AteBeva has not been firmly established. Moreover, clinicians have continued their attempts at multidisciplinary treatment that includes other systemic therapy and radiotherapy (RT). Here, we report a case that showed a near complete response (CR) of lung metastasis to nivolumab with ipilimumab therapy after achieving a near CR of intrahepatic tumor using sorafenib and RT in a patient with advanced HCC who had experienced treatment failure of AteBeva.
Keyword
Figure
Reference
-
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019; 380:1450–1462.
Article2. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.
Article3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article4. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC). 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2022; 23:1126–1240.5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article6. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.
Article7. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020; 6:e204564.8. Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021; 9:e001945.
Article9. Roessler D, Öcal O, Philipp AB, Markwardt D, Munker S, Mayerle J, et al. Ipilimumab and nivolumab in advanced hepatocellular carcinoma after failure of prior immune checkpoint inhibitor-based combination therapies: a multicenter retrospective study. J Cancer Res Clin Oncol. 2022; Jul. 21. . doi: 10.1007/s00432-022-04206-8. [Epub ahead of print].
Article10. Chu SS, Kuo YH, Liu WS, Wang SC, Ho CH, Chen YC, et al. Effect of radiotherapy on survival in advanced hepatocellular carcinoma patients treated with sorafenib: a nationwide cancer-registrybased study. Sci Rep. 2021; 11:1614.
Article11. Abulimiti M, Li Z, Wang H, Apiziaji P, Abulimiti Y, Tan Y. Combination intensity-modulated radiotherapy and sorafenib improves outcomes in hepatocellular carcinoma with portal vein tumor thrombosis. J Oncol. 2021; 2021:9943683.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- Complete response in hepatocellular carcinoma with lymph node metastasis by combination therapy of atezolizumab and bevacizumab: a case report
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- A Case of Transverse Myelitis Following Treatment with Atezolizumab for Advanced Hepatocellular Carcinoma
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report