J Liver Cancer.
2023 Mar;23(1):157-165. 10.17998/jlc.2023.02.07.
Diagnostic performance of the 2022 KLCA-NCC criteria for hepatocellular carcinoma on magnetic resonance imaging with extracellular contrast and hepatobiliary agents: comparison with the 2018 KLCA-NCC criteria
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Radiology, Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- KMID: 2540828
- DOI: http://doi.org/10.17998/jlc.2023.02.07
Abstract
- Background
/Aim: This study aimed to determine the diagnostic performance of 2022 Korean Liver Cancer Association-National Cancer Center (KLCA-NCC) imaging criteria compared with the 2018 KLCA-NCC for hepatocellular carcinoma (HCC) in high-risk patients using magnetic resonance imaging (MRI).
Methods
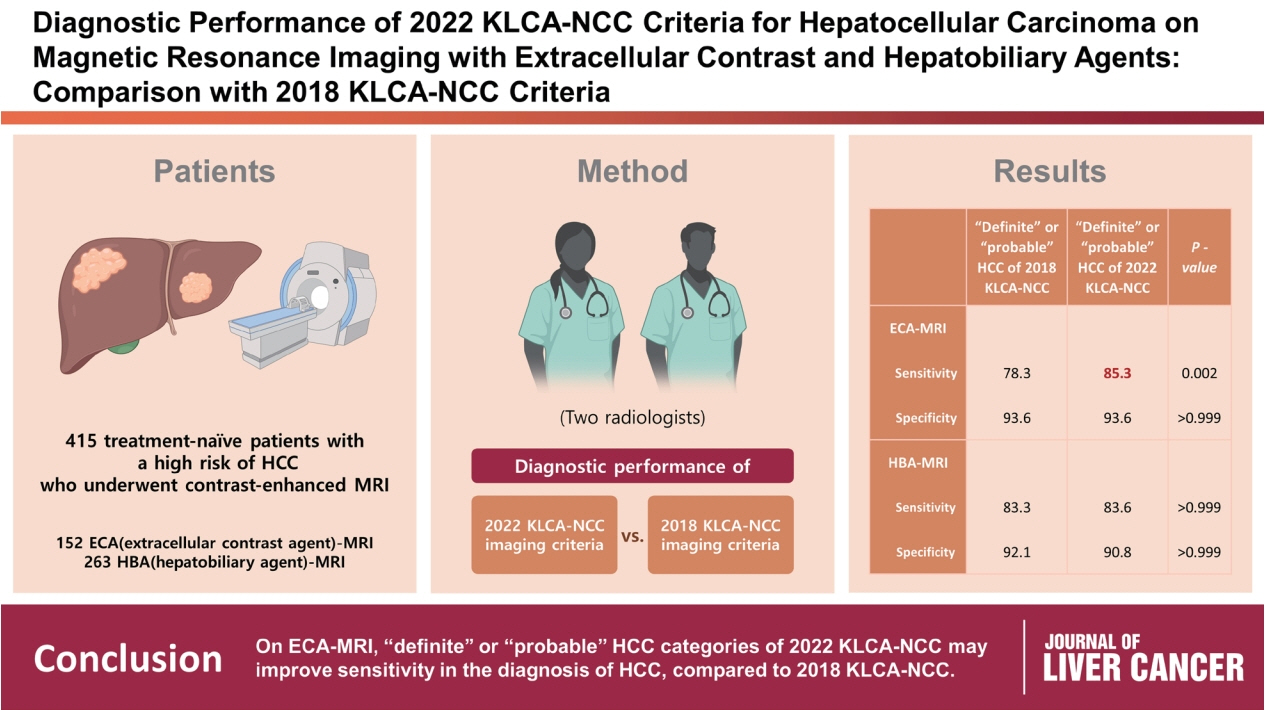
This retrospective study included 415 treatment-naïve patients (152 patients who underwent extracellular contrast agent [ECA]-MRI and 263 who underwent hepatobiliary agent [HBA]-MRI; 535 lesions, including 412 HCCs) with a high risk of HCC who underwent contrast-enhanced MRI. Two readers evaluated all lesions according to the 2018 and 2022 KLCA-NCC imaging diagnostic criteria, and the per-lesion diagnostic performances were compared.
Results
In “definite” HCC category of both 2018 and 2022 KLCA-NCC, HBA-MRI showed a significantly higher sensitivity for the diagnosis of HCC than ECA-MRI (77.0% vs. 64.3%, P=0.006) without a significant difference in specificity (94.7% vs. 95.7%, P=0.801). On ECAMRI, “definite” or “probable” HCC categories of the 2022 KLCA-NCC had significantly higher sensitivity than those of the 2018 KLCA-NCC (85.3% vs. 78.3%, P=0.002) with identical specificity (93.6%). On HBA-MRI, the sensitivity and specificity of “definite” or “probable” HCC categories of both 2018 and 2022 KLCA-NCC were not significantly different (83.3% vs. 83.6%, P>0.999 and 92.1% vs. 90.8%, P>0.999, respectively).
Conclusions
In “definite” HCC category of both 2018 and 2022 KLCA-NCC, HBA-MRI provides better sensitivity than ECA-MRI without compromising specificity. On ECA-MRI, “definite” or “probable” HCC categories of the 2022 KLCA-NCC may improve sensitivity in the diagnosis of HCC compared with the 2018 KLCA-NCC.
Figure
Cited by 2 articles
-
Impact of the updated KLCA-NCC criteria for diagnosis of “probable HCC” in liver MRI: comparisons between KLCA v2022 and v2018
Jeong Hee Yoon
J Liver Cancer. 2023;23(1):124-126. doi: 10.17998/jlc.2023.3.7.Performance of HCC diagnosis in the KLCA-NCC guidelines: a closer look at MRI contrast agents and HCC imaging hallmarks
Ji Hye Min, Young Kon Kim
J Liver Cancer. 2024;24(1):7-8. doi: 10.17998/jlc.2023.10.08.
Reference
-
References
1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019; 70:151–171.
Article2. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.
Article3. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.4. Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004; 10:88–98.5. Korean Liver Cancer Study Group and National Cancer Center. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009; 15:391–423.6. Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015; 9:267–317.7. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019; 13:227–299.8. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC). 2018 Korean Liver Cancer AssociationNational Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019; 20:1042–1113.9. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.10. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2022; 23:1126–1240.11. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023; 23:1–120.12. Choi SH, Byun JH, Lim YS, Yu E, Lee SJ, Kim SY, et al. Diagnostic criteria for hepatocellular carcinoma ≤3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol. 2016; 64:1099–1107.
Article13. Joo I, Lee JM, Lee DH, Jeon JH, Han JK. Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol. 2019; 29:1724–1732.
Article14. Kim DH, Choi SH, Kim SY, Kim MJ, Lee SS, Byun JH. Gadoxetic acid-enhanced MRI of hepatocellular carcinoma: value of washout in transitional and hepatobiliary phases. Radiology. 2019; 291:651–657.
Article15. Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019; 25:245–263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of the Korean Liver Cancer Association-National Cancer Center 2018 Criteria for the Noninvasive Diagnosis of Hepatocellular Carcinoma Using Magnetic Resonance Imaging
- Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging
- Emerging Role of Hepatobiliary Magnetic Resonance Contrast Media and Contrast-Enhanced Ultrasound for Noninvasive Diagnosis of Hepatocellular Carcinoma: Emphasis on Recent Updates in Major Guidelines
- Intraindividual Comparison of MRIs with Extracellular and Hepatobiliary Contrast Agents for the Noninvasive Diagnosis of Hepatocellular Carcinoma Using the Korean Liver Cancer Association–National Cancer Center 2022 Criteria
- Diagnostic Performance of LI-RADS v2018 versus KLCA-NCC 2018 Criteria for Hepatocellular Carcinoma Using Magnetic Resonance Imaging with Hepatobiliary Agent: A Systematic Review and Meta-Analysis of Comparative Studies