Kosin Med J.
2023 Mar;38(1):28-35. 10.7180/kmj.22.145.
Revascularization of immature retinas with retinopathy of prematurity using combination therapy of deferred laser treatment after a single intravitreal bevacizumab injection
- Affiliations
-
- 1Department of Ophthalmology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Ophthalmology, Chungnam National University Sejong Hospital, Sejong, Korea
- 3Department of Ophthalmology, Graduate School of Medicine, Pusan National University, Busan, Korea
- 4Department of Ophthalmology, Inje University College of Medicine, Busan, Korea
- KMID: 2540759
- DOI: http://doi.org/10.7180/kmj.22.145
Abstract
- Background
This study aimed to observe the extent of retinal vascularization in patients with retinopathy of prematurity (ROP) who underwent deferred laser treatment (LT) after a single intravitreal bevacizumab injection (IVB).
Methods
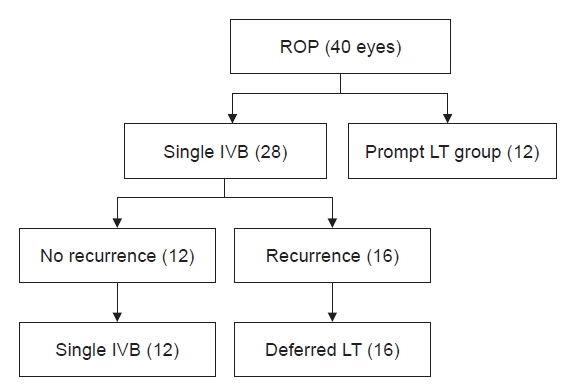
This study retrospectively evaluated 40 consecutive eyes in 21 infants who received a single IVB or LT. Deferred LT was performed in cases of ROP recurrence after a single IVB. To assess the amount of retinal vascularization between the initial IVB and deferred LT, the cases were divided into three groups based on treatment: single IVB, deferred LT after a single IVB, and prompt LT. The growth and associated complications were compared between groups.
Results
There were 12, 16, and 12 eyes in the single IVB, deferred LT, and prompt LT groups, respectively. Deferred LT was performed at an average of 7.9 weeks after a single IVB. In the single IVB group, retinal vascularization proceeded to zone III, whereas the prompt LT group did not show any growth of vascularization beyond the laser scars. In the deferred LT group, during the window period before LT, retinal vascularization progressed from zone I to zone II posterior and from zone II posterior to zone II anterior, respectively, without further ROP recurrence.
Conclusions
Retinal vascularization progressed during the deferred window period, thereby reducing the area of the retina ablated by LT. A single IVB followed by deferred LT can be an alternative treatment option to prevent ablation of zone I or multiple IVBs.
Figure
Reference
-
References
1. Hiraoka M, Watanabe T, Kawakami T, Ito R, Takigawa I, Suzumura H, et al. Retinopathy of prematurity in extremely low birth weight infants: a Tokyo multicenter study. Nippon Ganka Gakkai Zasshi. 2004; 108:600–5.2. Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004; 102:233–50.3. Wu WC, Yeh PT, Chen SN, Yang CM, Lai CC, Kuo HK. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in Taiwan. Ophthalmology. 2011; 118:176–83.4. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364:603–15.5. Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015; 122:1008–15.6. Dorta P, Kychenthal A. Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina. 2010; 30(4 Suppl):S24–31.7. Hoang QV, Kiernan DF, Chau FY, Shapiro MJ, Blair MP. Fluorescein angiography of recurrent retinopathy of prematurity after initial intravitreous bevacizumab treatment. Arch Ophthalmol. 2010; 128:1080–1.8. Kong L, Mintz-Hittner HA, Penland RL, Kretzer FL, Chevez-Barrios P. Intravitreous bevacizumab as anti-vascular endothelial growth factor therapy for retinopathy of prematurity: a morphologic study. Arch Ophthalmol. 2008; 126:1161–3.9. Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015; 133:391–7.10. Hong YR, Kim YH, Kim SY, Nam GY, Cheon HJ, Lee SJ. Plasma concentrations of vascular endothelial growth factor in retinopathy of prematurity after intravitreal bevacizumab injection. Retina. 2015; 35:1772–7.11. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1727–30.12. Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, et al. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings to 3 years of age. Ophthalmology. 2008; 115:1058–64. e1.13. Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014; 132:1327–33.14. Yoon JM, Shin DH, Kim SJ, Ham DI, Kang SW, Chang YS, et al. Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina. 2017; 37:88–96.15. Tandon M, Vishal MY, Kohli P, Rajan RP, Ramasamy K. Supplemental laser for eyes treated with bevacizumab monotherapy in severe retinopathy of prematurity. Ophthalmol Retina. 2018; 2:623–8.16. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003; 121:1684–94.17. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005; 123:991–9.18. Mintz-Hittner HA, Kuffel RR. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008; 28:831–8.19. Mintz-Hittner HA, Best LM. Antivascular endothelial growth factor for retinopathy of prematurity. Curr Opin Pediatr. 2009; 21:182–7.20. O'Connor A, Fielder AR. Long term ophthalmic sequelae of prematurity. Early Hum Dev. 2008; 84:101–6.21. Yang CS, Wang AG, Sung CS, Hsu WM, Lee FL, Lee SM. Long-term visual outcomes of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye (Lond). 2010; 24:14–20.22. McLoone EM, O'Keefe M, McLoone SF, Lanigan BM. Long-term refractive and biometric outcomes following diode laser therapy for retinopathy of prematurity. J AAPOS. 2006; 10:454–9.23. Ling KP, Liao PJ, Wang NK, Chao AN, Chen KJ, Chen TL, et al. Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina. 2020; 40:1793–803.24. Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012; 130:1000–6.25. Nazari H, Modarres M, Parvaresh MM, Ghasemi Falavarjani K. Intravitreal bevacizumab in combination with laser therapy for the treatment of severe retinopathy of prematurity (ROP) associated with vitreous or retinal hemorrhage. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1713–8.26. Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, et al. Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012; 153:327–33. e1.27. Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2015; 56:956–61.28. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.29. Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009; 6:465–77.30. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006; 290:H560–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Primary Intravitreal Ranibizumab Injection for Treatment of Type 1 Retinopathy of Prematurity
- Effect of Laser Photocoagulation and Intravitreal Bevacizumab Injection on Zone I Retinopathy of Prematurity
- Letter to the Editor: Effect of Intravitreal Bevacizumab Injection on Retinopathy of Prematurity
- Effect of Primary Intravitreal Bevacizumab Injection on Stage 3 Retinopathy of Prematurity with Plus Signs
- Comparison of Intravitreal Bevacizumab and Ranibizumab Injections in Aggressive and Type 1 Retinopathy of Prematurity