J Korean Ophthalmol Soc.
2015 Jan;56(1):62-69. 10.3341/jkos.2015.56.1.62.
Effect of Primary Intravitreal Bevacizumab Injection on Stage 3 Retinopathy of Prematurity with Plus Signs
- Affiliations
-
- 1Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. sklee219@yuhs.ac
- KMID: 2216155
- DOI: http://doi.org/10.3341/jkos.2015.56.1.62
Abstract
- PURPOSE
To evaluate the efficacy and safety of primary intravitreal bevacizumab injection in stage 3 retinopathy of prematurity with plus signs.
METHODS
We reviewed retrospectively the medical records of 30 eyes of 16 patients diagnosed with stage 3 retinopathy of prematurity with plus signs treated with primary intravitreal bevacizumab injection between March 1, 2011 and February 28, 2013 and followed up for at least 9 months.
RESULTS
Mean gestational age was 26 + 4 weeks +/- 11 days and mean birth weight was 822 +/- 251.4 g. The locations of disease were zone II in 24 eyes and zone III in 6 eyes. Intravitreal bevacizumab injection was performed after the mean 1.3 +/- 1 day after plus signs were detected. Mean postconceptional age at treatment was 38 + 2 weeks +/- 16 days. Mean follow-up period was 16.6 +/- 6.9 months. Plus signs started to regress after the mean 4.6 +/- 2.3 days after injection and completely regressed after the mean 24.3 +/- 12.4 days. Cataract extraction was performed in 1 eye due to a cataract that appeared not associated with the injection procedure, but was regarded as a treatment failure. There were no local or systemic complications.
CONCLUSIONS
Primary intravitreal bevacizumab injection in stage 3 retinopathy of prematurity with plus signs demonstrated excellent short-term efficacy and safety.
Keyword
MeSH Terms
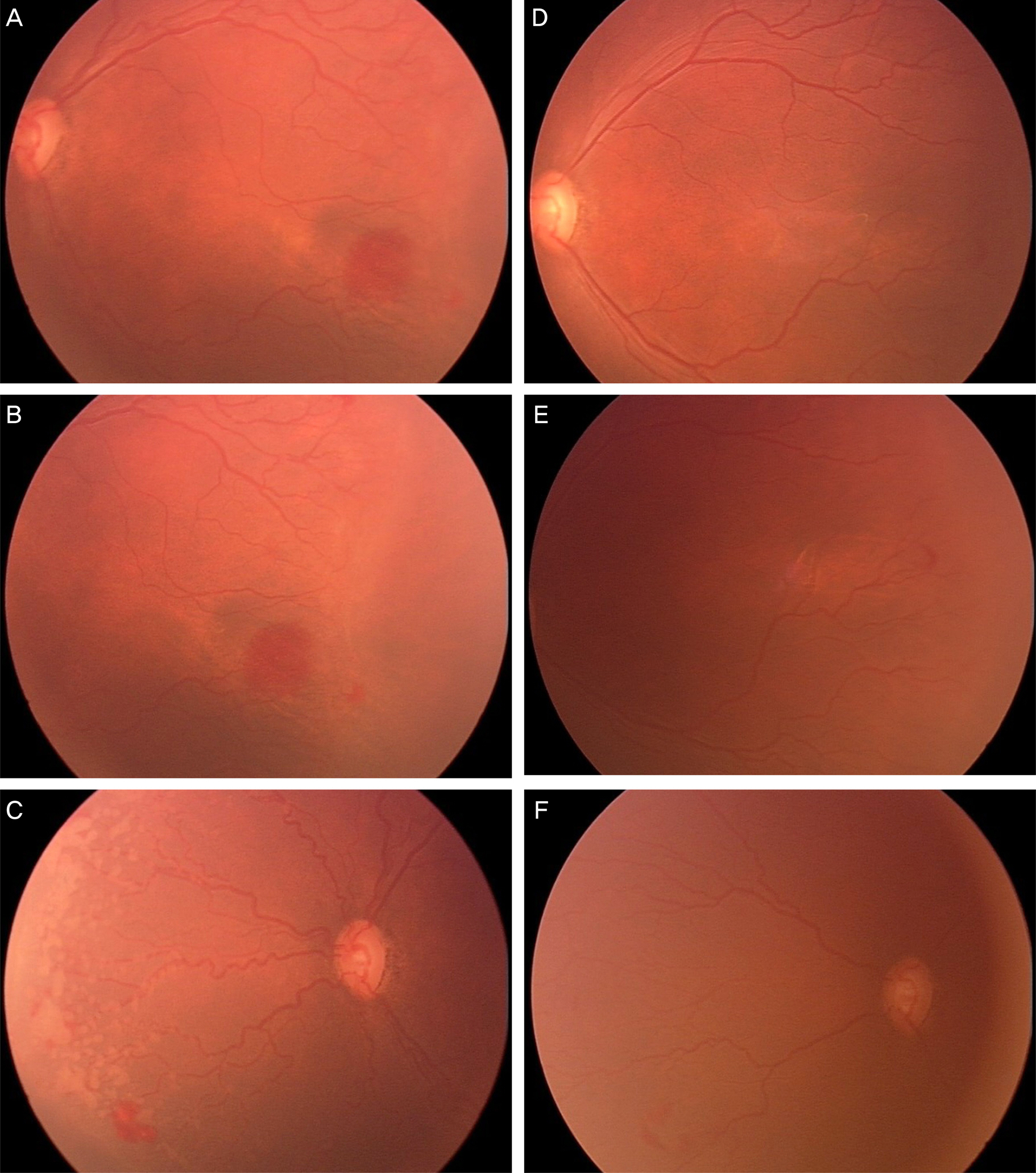
Figure
Cited by 2 articles
-
Letter to the Editor: Effect of Intravitreal Bevacizumab Injection on Retinopathy of Prematurity
YC Kim
J Korean Ophthalmol Soc. 2015;56(10):1650-1650. doi: 10.3341/jkos.2015.56.10.1650.Efficacy of Primary Intravitreal Ranibizumab Injection for Treatment of Type 1 Retinopathy of Prematurity
Gyu Chul Chung, Sung-Hyuk Moon
J Korean Ophthalmol Soc. 2017;58(9):1080-1086. doi: 10.3341/jkos.2017.58.9.1080.
Reference
-
References
1. Terry TL. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Trans Am Ophthalmol Soc. 1942; 40:262–84.2. Darlow BA, Hutchinson JL, Henderson-Smart DJ. . Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005; 115:990–6.
Article3. Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004; 24:164–8.
Article4. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Carlo WA, Finer NN. . Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010; 362:1959–69.
Article5. Tompkins C. A sudden rise in the prevalence of retinopathy of prematurity blindness? Pediatrics. 2001; 108:526.
Article6. Gibson DL, Sheps SB, Schechter MT. . Retinopathy of pre-maturity: a new epidemic? Pediatrics. 1989; 83:486–92.
Article7. Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008; 49:5177–82.
Article8. Das A, McGuire PG. Retinal and choroidal angiogenesis: patho-physiology and strategies for inhibition. Prog Retin Eye Res. 2003; 22:721–48.
Article9. Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of pre-maturity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003; 121:1684–94.10. Good WV. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004; 102:233–48. discussion 248-50.11. Hurwitz H, Fehrenbacher L, Novotny W. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.
Article12. CATT Research Group, Martin DF, Maguire MG. . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–908.
Article13. Tonello M, Costa RA, Almeida FP. . Panretinal photo-coagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol. 2008; 86:385–9.
Article14. Michaelides M, Kaines A, Hamilton RD. . A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010; 117:1078–86.e2.15. Epstein DL, Algvere PV, von Wendt G. . Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical study. Ophthalmology. 2012; 119:1184–9.
Article16. Higashiyama T, Sawada O, Kakinoki M. . Prospective comparisons of intravitreal injections of triamcinolone acetonide and bevacizumab for macular oedema due to branch retinal vein occlusion. Acta Ophthalmol. 2013; 91:318–24.
Article17. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123:991–9.18. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome--structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1990; 108:1408–16.19. Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology. 2009; 116:1599–603.
Article20. Lee JY, Chae JB, Yang SJ. . Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1257–62.
Article21. Choi W, Heo H. Effect of laser photocoagulation and intravitreal bevacizumab injection on zone I retinopathy of prematurity. J Korean Ophthalmol Soc. 2012; 53:120–6.
Article22. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364:603–15.
Article23. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intra-vitreous injection: a comprehensive review. Retina. 2004; 24:676–98.
Article24. Kodjikian L, Souied EH, Mimoun G. . Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013; 120:2300–9.
Article25. Wu WC, Yeh PT, Chen SN. . Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in taiwan. Ophthalmology. 2011; 118:176–83.
Article26. Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bev-acizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008; 28:831–8.
Article27. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photo-coagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1727–30.
Article28. Sato T, Wada K, Arahori H. . Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012; 153:327–33.e1.
Article29. Karaca C, Oner AO, Mirza E. . Bilateral effect of unilateral bevacizumab injection in retinopathy of prematurity. JAMA Ophthalmol. 2013; 131:1099–101.
Article30. Larsen JS. The sagittal growth of the eye. 3. Ultrasonic measurement of the posterior segment (axial length of the vitreous) from birth to puberty. Acta Ophthalmol (Copenh). 1971; 49:441–53.31. Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal lowdosage bevacizumab for retinopathy of prematurity. Acta Ophthalmol. 2014; 92:577–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor: Effect of Intravitreal Bevacizumab Injection on Retinopathy of Prematurity
- Efficacy of Primary Intravitreal Ranibizumab Injection for Treatment of Type 1 Retinopathy of Prematurity
- Effect of Laser Photocoagulation and Intravitreal Bevacizumab Injection on Zone I Retinopathy of Prematurity
- Comparison of Intravitreal Bevacizumab and Ranibizumab Injections in Aggressive and Type 1 Retinopathy of Prematurity
- Short-term Effect of Intravitreal Bevacizumab Injection Preventing Panretinal Photocoagulation-Induced Macular Edema in Proliferative Diabetic Retinopathy