Diabetes Metab J.
2023 Mar;47(2):287-300. 10.4093/dmj.2021.0306.
N6-Methyladenosine Methyltransferase METTL3 Alleviates Diabetes-Induced Testicular Damage through Modulating TUG1/Clusterin Axis
- Affiliations
-
- 1Department of Urology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 2School of Clinical Medicine, Guizhou Medical University, Guiyang, China
- KMID: 2540524
- DOI: http://doi.org/10.4093/dmj.2021.0306
Abstract
- Background
The present study investigated the regulatory effects of N6-methyladenosine (m6A) methyltransferase like-3 (METTL3) in diabetes-induced testicular damage.
Methods
In vivo diabetic mice and high glucose (HG) treated GC-1 spg cells were established. The mRNA and protein expressions were determined by real-time quantitative polymerase chain reaction, Western blot, immunofluorescence and immunohistochemistry staining. Levels of testosterone, blood glucose, cell viability, and apoptosis were detected by enzyme-linked immunosorbent assay, MTT, and flow cytometry, respectively. Molecular interactions were verified by RNA immunoprecipitation and RNA pull-down assay. Histopathological staining was performed to evaluate testicular injury.
Results
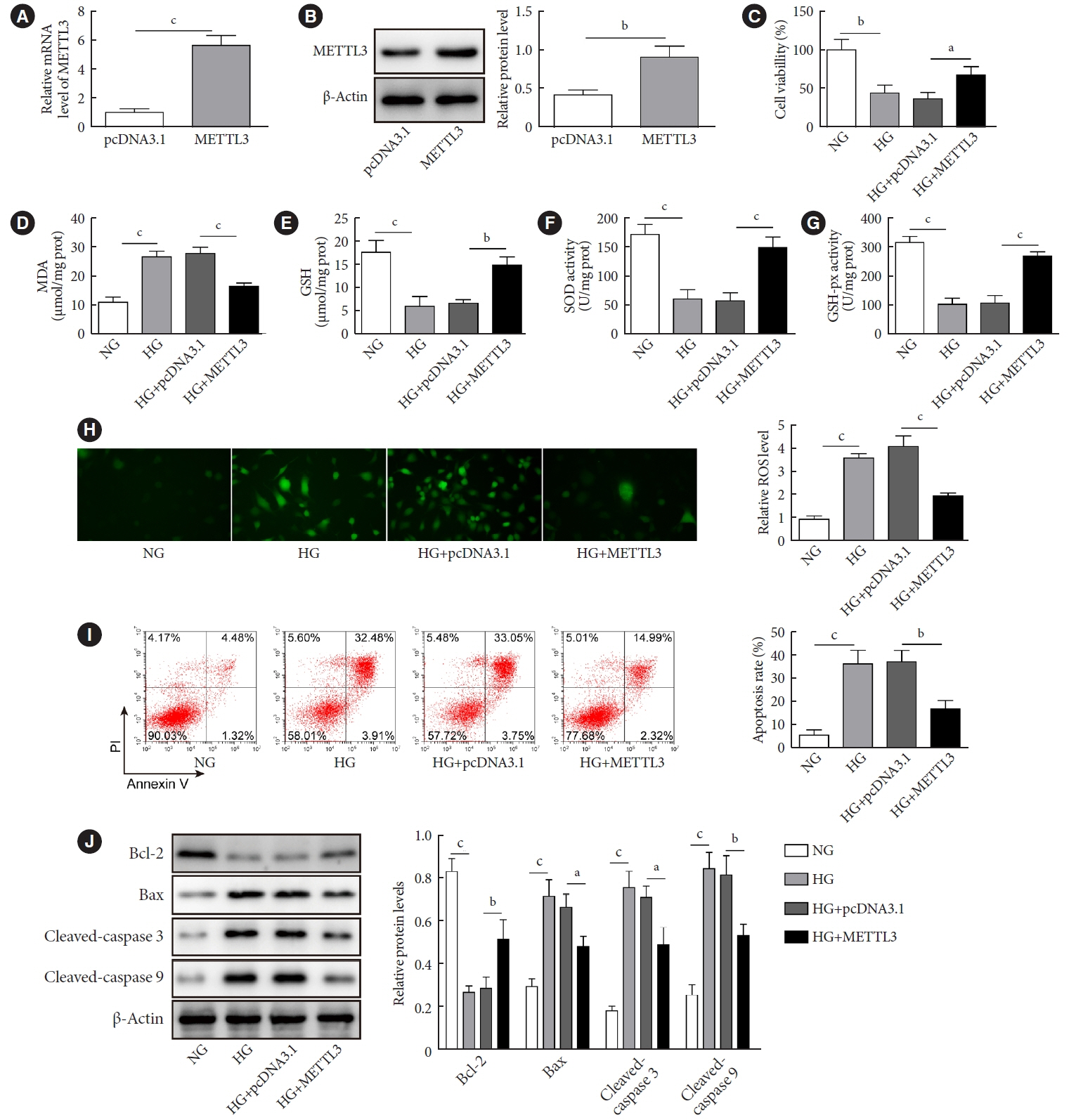
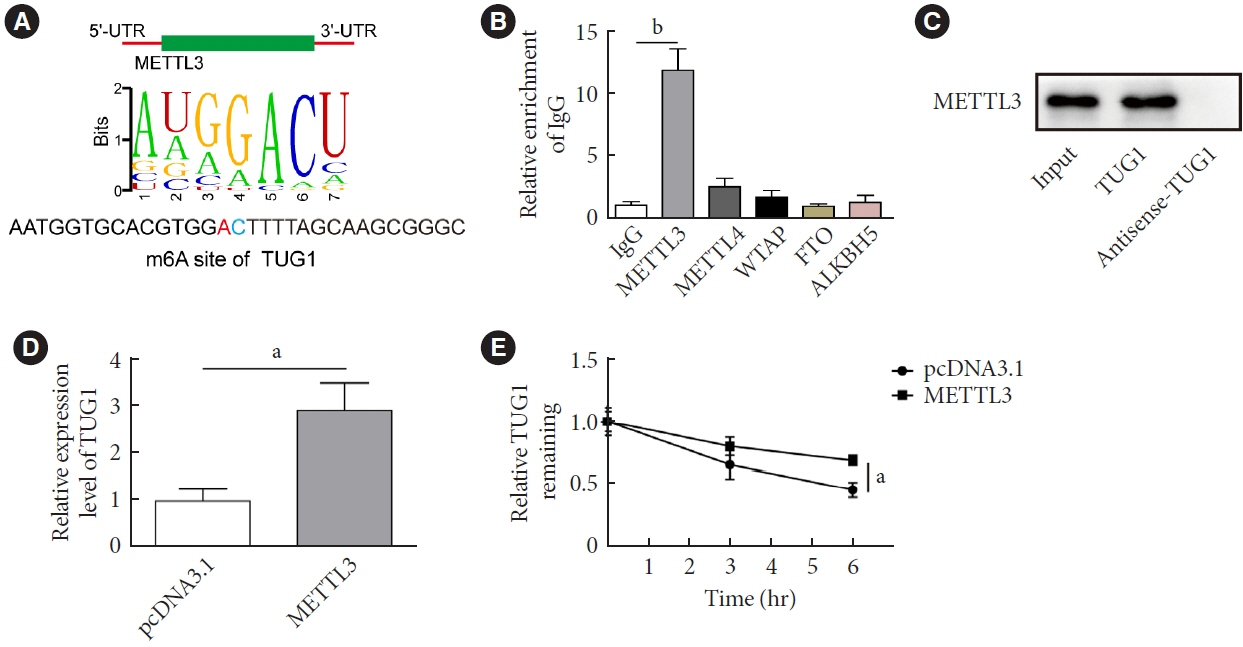
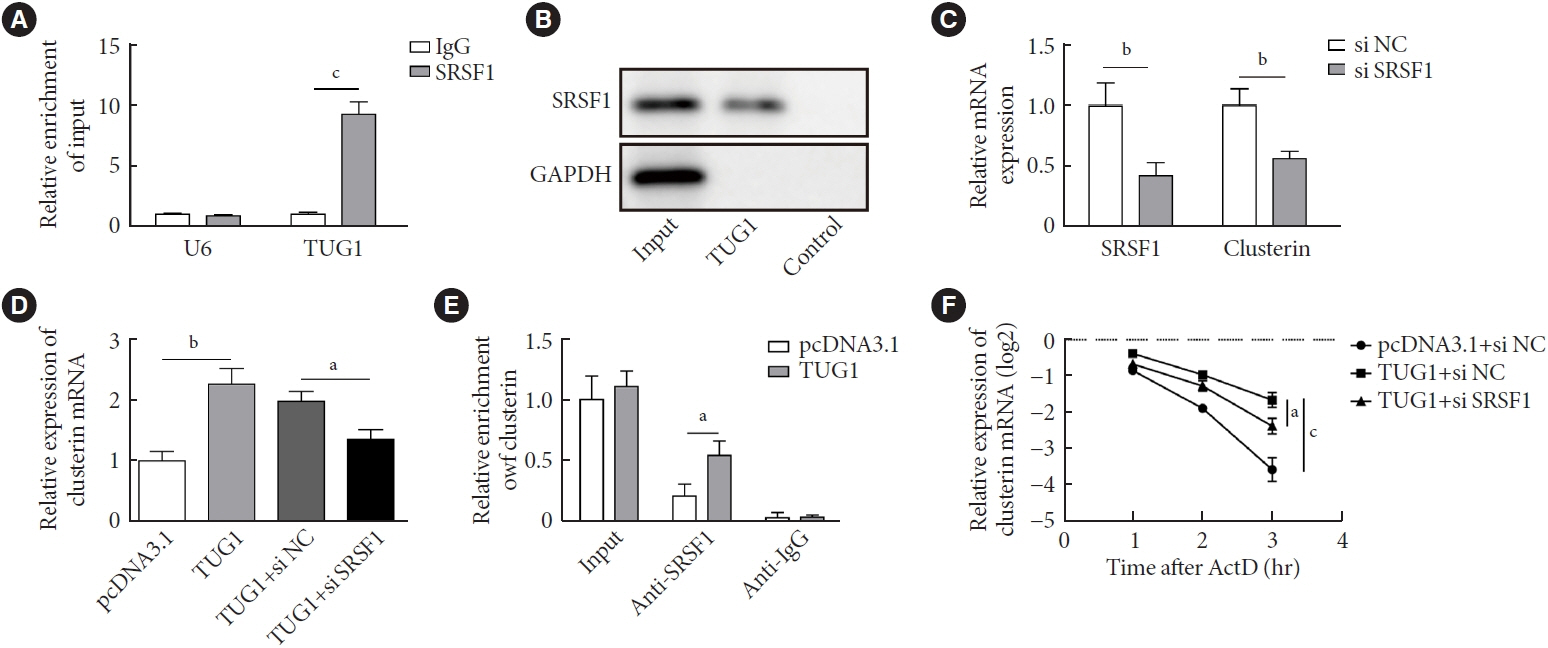
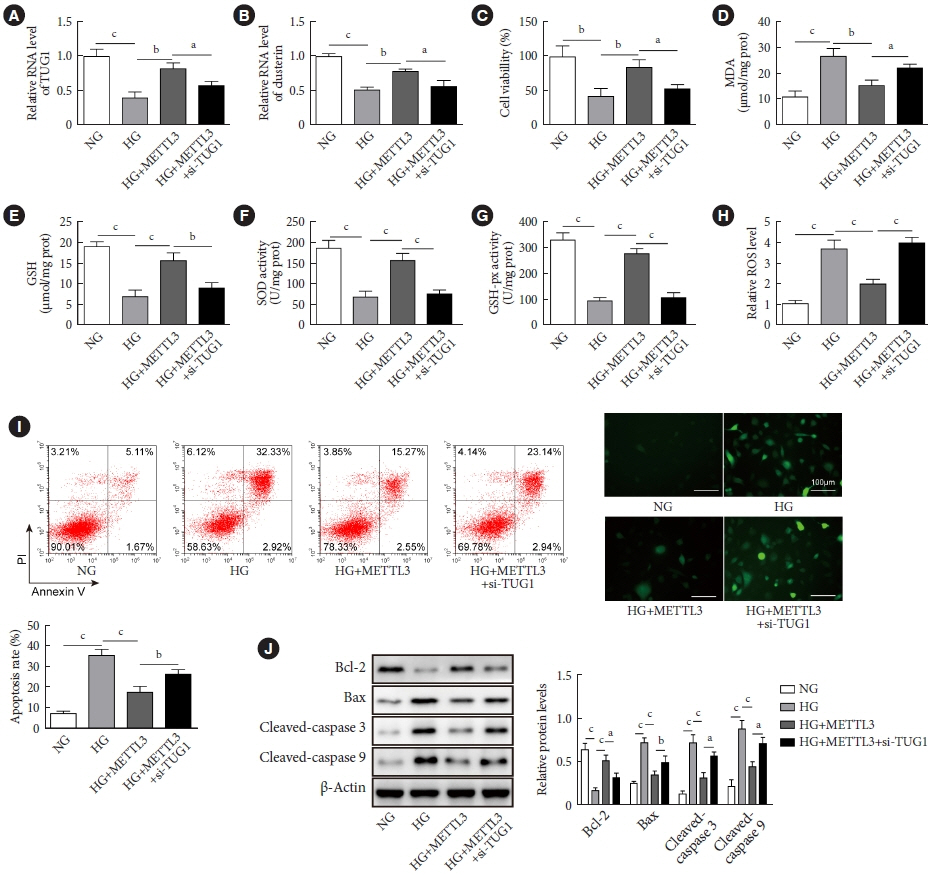
METTL3 and long non-coding RNA taurine up-regulated 1 (lncRNA TUG1) were downregulated in testicular tissues of diabetic mice and HG-treated GC-1 spg cells. METTL3 overexpression could reduce the blood glucose level, oxidative stress and testicular damage but enhance testosterone secretion in diabetic mouse model and HG-stimulated GC-1 spg cells. Mechanically, METTL3-mediated m6A methylation enhanced the stability of TUG1, then stabilizing the clusterin mRNA via recruiting serine and arginine rich splicing factor 1. Moreover, inhibition of TUG1/clusterin signaling markedly reversed the protective impacts of METTL3 overexpression on HG-stimulated GC-1 spg cells.
Conclusion
This study demonstrated that METTL3 ameliorated diabetes-induced testicular damage by upregulating the TUG1/clusterin signaling. These data further elucidate the potential regulatory mechanisms of m6A modification on diabetes-induced testicular injury.
Figure
Cited by 1 articles
-
Rbbp6-Mediated Bmal1 Ubiquitination Inhibits YAP1 Signaling Pathway to Promote Ferroptosis in Diabetes-Induced Testicular Damage
Yuan Tian, Zhiqiang Zhu, Jun Qiao, Bei Liu, Yuehai Xiao
Diabetes Metab J. 2025;49(2):210-224. doi: 10.4093/dmj.2024.0099.
Reference
-
1. Liu Y, Yang Z, Kong D, Zhang Y, Yu W, Zha W. Metformin ameliorates testicular damage in male mice with streptozotocin-induced type 1 diabetes through the PK2/PKR pathway. Oxid Med Cell Longev. 2019; 2019:5681701.2. Ghazi S, Zohdy W, Elkhiat Y, Shamloul R. Serum testosterone levels in diabetic men with and without erectile dysfunction. Andrologia. 2012; 44:373–80.3. Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007; 22:1871–7.4. Long L, Qiu H, Cai B, Chen N, Lu X, Zheng S, et al. Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget. 2018; 9:5321–36.5. Zhao Y, Chen Y, Jin M, Wang J. The crosstalk between m6A RNA methylation and other epigenetic regulators: a novel perspective in epigenetic remodeling. Theranostics. 2021; 11:4549–66.6. Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021; 6:74.7. Huang H, Weng H, Chen J. m6A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020; 37:270–88.8. Yang J, Liu J, Zhao S, Tian F. N(6)-Methyladenosine METTL3 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract. Mol Ther Nucleic Acids. 2020; 20:111–6.9. Zha X, Xi X, Fan X, Ma M, Zhang Y, Yang Y. Overexpression of METTL3 attenuates high-glucose induced RPE cell pyroptosis by regulating miR-25-3p/PTEN/Akt signaling cascade through DGCR8. Aging (Albany NY). 2020; 12:8137–50.10. Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014; 5:e1506.11. Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X, et al. LncRNA KCNQ1OT1 mediates pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem. 2018; 50:1230–44.12. Long J, Badal SS, Ye Z, Wang Y, Ayanga BA, Galvan DL, et al. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016; 126:4205–18.13. Li Y, Zhi K, Han S, Li X, Li M, Lian W, et al. TUG1 enhances high glucose-impaired endothelial progenitor cell function via miR-29c-3p/PDGF-BB/Wnt signaling. Stem Cell Res Ther. 2020; 11:441.14. Wilson MR, Zoubeidi A. Clusterin as a therapeutic target. Expert Opin Ther Targets. 2017; 21:201–13.15. Ren L, Han F, Xuan L, Lv Y, Gong L, Yan Y, et al. Clusterin ameliorates endothelial dysfunction in diabetes by suppressing mitochondrial fragmentation. Free Radic Biol Med. 2019; 145:357–73.16. He J, Dijkstra KL, Bakker K, Bus P, Bruijn JA, Scharpfenecker M, et al. Glomerular clusterin expression is increased in diabetic nephropathy and protects against oxidative stress-induced apoptosis in podocytes. Sci Rep. 2020; 10:14888.17. Tian Y, Xiao YH, Geng T, Sun C, Gu J, Tang KF, et al. Clusterin suppresses spermatogenic cell apoptosis to alleviate diabetesinduced testicular damage by inhibiting autophagy via the PI3K/AKT/mTOR axis. Biol Cell. 2021; 113:14–27.18. Verma P, Parte P. Revisiting the characteristics of testicular germ cell lines GC-1(spg) and GC-2(spd)ts. Mol Biotechnol. 2021; 63:941–52.19. Zheng H, Huang J, Zhang M, Zhao HJ, Chen P, Zeng ZH. miR-27b-3p improved high glucose-induced spermatogenic cell damage via regulating Gfpt1/HBP signaling. Eur Surg Res. 2022; 63:64–76.20. Liao J, Xiao H, Dai G, He T, Huang W. Recombinant adenovirus (AdEasy system) mediated exogenous expression of long non-coding RNA H19 (lncRNA H19) biphasic regulating osteogenic differentiation of mesenchymal stem cells (MSCs). Am J Transl Res. 2020; 12:1700–13.21. Rastogi RP, Singh SP, Hader DP, Sinha RP. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2’,7’-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun. 2010; 397:603–7.22. De Jesus DF, Zhang Z, Kahraman S, Brown NK, Chen M, Hu J, et al. m6A mRNA methylation regulates human β-cell biology in physiological states and in type 2 diabetes. Nat Metab. 2019; 1:765–74.23. Liu J, Luo G, Sun J, Men L, Ye H, He C, et al. METTL14 is essential for β-cell survival and insulin secretion. Biochim Biophys Acta Mol Basis Dis. 2019; 1865:2138–48.24. Kobayashi M, Ohsugi M, Sasako T, Awazawa M, Umehara T, Iwane A, et al. The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol Cell Biol. 2018; 38:e00116–18.25. Li Y, Zhang Q, Cui G, Zhao F, Tian X, Sun BF, et al. m6A regulates liver metabolic disorders and hepatogenous diabetes. Genomics Proteomics Bioinformatics. 2020; 18:371–83.26. Lin Z, Hsu PJ, Xing X, Fang J, Lu Z, Zou Q, et al. Mettl3-/Mettl14-mediated mRNA N6-methyladenosine modulates murine spermatogenesis. Cell Res. 2017; 27:1216–30.27. Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017; 549:273–6.28. Wang J, Ishfaq M, Xu L, Xia C, Chen C, Li J. METTL3/m6A/ miRNA-873-5p attenuated oxidative stress and apoptosis in colistin-induced kidney injury by modulating Keap1/Nrf2 pathway. Front Pharmacol. 2019; 10:517.29. Li X, Jiang Y, Sun X, Wu Y, Chen Z. METTL3 is required for maintaining β-cell function. Metabolism. 2021; 116:154702.30. Kapucu A, Akgun-Dar K. Leptin ameliorates testicular injury by altering expression of nitric oxide synthases in diabetic rats. Bratisl Lek Listy. 2021; 122:111–5.31. Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019; 18:142.32. Chang YZ, Chai RC, Pang B, Chang X, An SY, Zhang KN, et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-κB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett. 2021; 511:36–46.33. Zhang Y, Ma Y, Gu M, Peng Y. lncRNA TUG1 promotes the brown remodeling of white adipose tissue by regulating miR-204-targeted SIRT1 in diabetic mice. Int J Mol Med. 2020; 46:2225–34.34. Yan HY, Bu SZ, Zhou WB, Mai YF. TUG1 promotes diabetic atherosclerosis by regulating proliferation of endothelial cells via Wnt pathway. Eur Rev Med Pharmacol Sci. 2018; 22:6922–9.35. Zhao L, Li W, Zhao H. Inhibition of long non-coding RNA TUG1 protects against diabetic cardiomyopathy induced diastolic dysfunction by regulating miR-499-5p. Am J Transl Res. 2020; 12:718–30.36. Kim C, Kang D, Lee EK, Lee JS. Long noncoding RNAs and RNA-binding proteins in oxidative stress, cellular senescence, and age-related diseases. Oxid Med Cell Longev. 2017; 2017:2062384.37. Tang S, Zhu W, Zheng F, Gui W, Zhang W, Lin X, et al. The long noncoding RNA Blnc1 protects against diet-induced obesity by promoting mitochondrial function in white fat. Diabetes Metab Syndr Obes. 2020; 13:1189–201.38. Hu M, Wang R, Li X, Fan M, Lin J, Zhen J, et al. LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose-induced podocyte injury via its interplay with β-catenin. J Cell Mol Med. 2017; 21:2732–47.39. Tian Y, Xiao Y, Wang B, Sun C, Tang K, Sun F. Vitamin E and lycopene reduce coal burning fluorosis-induced spermatogenic cell apoptosis via oxidative stress-mediated JNK and ERK signaling pathways. Biosci Rep. 2018; 38:BSR20171003.40. Wittwer J, Bradley D. Clusterin and its role in insulin resistance and the cardiometabolic syndrome. Front Immunol. 2021; 12:612496.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- m6A Methyltransferase METTL3 Reduces Hippocampal Neuron Apoptosis in a Mouse Model of Autism Through the MALAT1/SFRP2/Wnt/β-catenin Axis
- METTL3 Affects Spinal Cord Neuronal Apoptosis by Regulating Bcl-2 m6A Modifications After Spinal Cord Injury
- STM2457 Inhibits METTL3-Mediated m6A Modification of miR-30c to Alleviate Spinal Cord Injury by Inducing the ATG5-Mediated Autophagy
- The Role of Clusterin in Apoptosis Induced by Testicular Torsion
- Rbbp6-Mediated Bmal1 Ubiquitination Inhibits YAP1 Signaling Pathway to Promote Ferroptosis in Diabetes-Induced Testicular Damage