J Korean Med Sci.
2023 Mar;38(10):e78. 10.3346/jkms.2023.38.e78.
Sudden Death Associated With Possible Flare-Ups of Multiple Sclerosis After COVID-19 Vaccination and Infection: A Case Report and Literature Review
- Affiliations
-
- 1Department of Forensic Medicine, Defense Institute of Forensic Science, Criminal Investigation Command, Ministry of National Defense, Seoul, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2540428
- DOI: http://doi.org/10.3346/jkms.2023.38.e78
Abstract
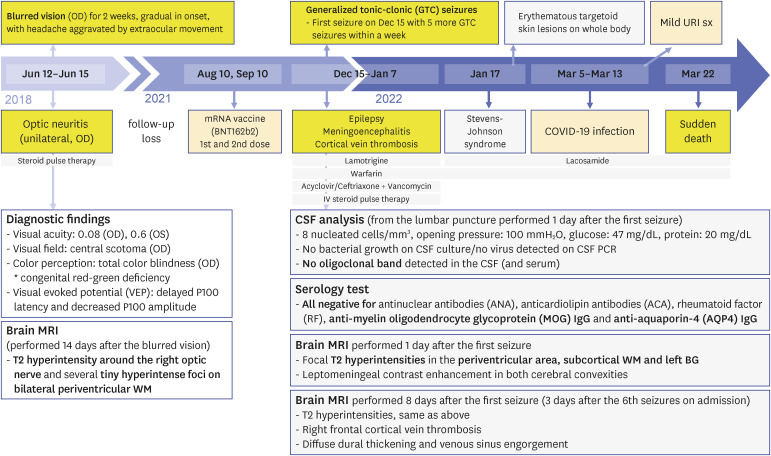
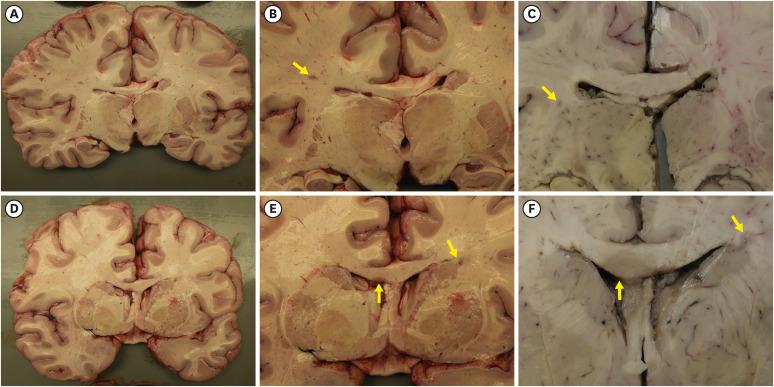
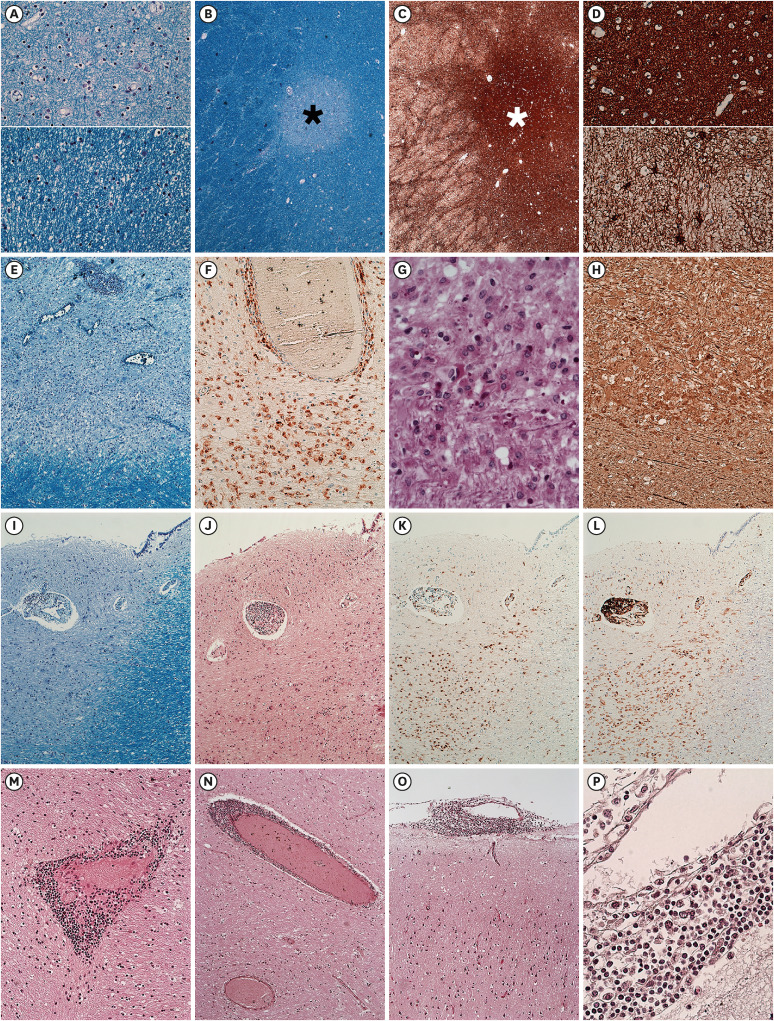
- We present an autopsy case of a 19-year-old man with a history of epilepsy whose unwitnessed sudden death occurred unexpectedly in the night. About 4 years before death, he was diagnosed with unilateral optic neuritis (ON). Demyelinating disease was suspected, but he was lost to follow up after the recovery. Six months before death, he received a second dose of mRNA coronavirus disease 2019 (COVID-19) vaccine. Three months before death, he experienced epileptic seizures for the first time. Seventeen days before death, he was infected with COVID-19, which showed self-limited course under home isolation. Several days before death, he complained of seizures again at night. Autopsy revealed multifocal gray-tan discoloration in the cerebrum. Histologically, the lesions consisted of active and inactive demyelinated plaques in the perivenous area of the white matter. Perivascular lymphocytic infiltration and microglial cell proliferation were observed in both white matter and cortex. The other major organs including heart and lung were unremarkable. Based on the antemortem history and postmortem findings, the cause of death was determined to be multiple sclerosis with suspected exacerbation. The direct or indirect involvement of cortex and deep gray matter by exacerbated multiple sclerosis may explain the occurrence of seizures. Considering the absence of other structural abnormalities except the inflammatory demyelination of the cerebrum, fatal arrhythmia or laryngospasm in the terminal epileptic seizure may explain his sudden unexpected death in the benign circumstances. In this case, the onset of seizure was preceded by COVID-19 vaccination, and the exacerbation of seizure was preceded by COVID-19 infection, respectively. Literature reporting first manifestation or relapse of multiple sclerosis temporally associated with COVID-19 vaccination or infection are reviewed.
Keyword
Figure
Cited by 1 articles
-
Sudden Death Is More Likely to Result From SARS-COV-2 Infection Than Multiple Sclerosis
Josef Finsterer
J Korean Med Sci. 2023;38(44):e393. doi: 10.3346/jkms.2023.38.e393.
Reference
-
1. Fragoso YD, Gomes S, Gonçalves MV, Mendes Junior E, Oliveira BE, Rocha CF, et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult Scler Relat Disord. 2022; 57:103321. PMID: 35158439.2. Fujimori J, Miyazawa K, Nakashima I. Initial clinical manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neuroimmunol. 2021; 361:577755. PMID: 34700047.3. Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022; 269(1):55–58. PMID: 34115170.4. Ismail II, Al-Hashel J, Alroughani R, Ahmed SF. A case report of multiple sclerosis after COVID-19 infection: causality or coincidence? Neuroimmunol Rep. 2021; 1:100008.5. Karsidag S, Sahin S, Ates MF, Cinar N, Kendirli S. Demyelinating disease of the central nervous system concurrent with COVID-19. Cureus. 2021; 13(8):e17297. PMID: 34552833.6. Kataria S, Rogers S, Bilal U, Baktashi H, Singh R. Multiple sclerosis relapse following COVID-19 vaccination: a case report and literature review. Cureus. 2022; 14(1):e21374. PMID: 35198286.7. Khayat-Khoei M, Bhattacharyya S, Katz J, Harrison D, Tauhid S, Bruso P, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2022; 269(3):1093–1106. PMID: 34480607.8. Maniscalco GT, Manzo V, Di Battista ME, Salvatore S, Moreggia O, Scavone C, et al. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report. Front Neurol. 2021; 12:721502. PMID: 34447349.9. Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021; 22:100299. PMID: 33313429.10. Nistri R, Barbuti E, Rinaldi V, Tufano L, Pozzilli V, Ianniello A, et al. Case report: multiple sclerosis relapses after vaccination against SARS-CoV2: a series of clinical cases. Front Neurol. 2021; 12:765954. PMID: 34744992.11. Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020; 45:102377. PMID: 32698095.12. Sarwar S, Rogers S, Mohamed AS, Ogula E, Ayantayo RA, Ahmed A, et al. Multiple sclerosis following SARS-CoV-2 infection: a case report and literature review. Cureus. 2021; 13(10):e19036. PMID: 34858736.13. Tagliaferri AR, Horani G, Stephens K, Michael P. A rare presentation of undiagnosed multiple sclerosis after the COVID-19 vaccine. J Community Hosp Intern Med Perspect. 2021; 11(6):772–775. PMID: 34804388.14. Toljan K, Amin M, Kunchok A, Ontaneda D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J Neuroimmunol. 2022; 362:577785. PMID: 34922126.15. Yavari F, Raji S, Moradi F, Saeidi M. Demyelinating changes alike to multiple sclerosis: a case report of rare manifestations of COVID-19. Case Rep Neurol Med. 2020; 2020:6682251. PMID: 33425411.16. Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien). 2020; 162(7):1491–1494. PMID: 32367205.17. Popescu BF, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum (Minneap Minn). 2013; 19(4 Multiple Sclerosis):901–921. PMID: 23917093.18. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17(2):162–173. PMID: 29275977.19. Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016; 15(3):292–303. PMID: 26822746.20. Chen L, Gordon LK. Ocular manifestations of multiple sclerosis. Curr Opin Ophthalmol. 2005; 16(5):315–320. PMID: 16175046.21. Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006; 67(2):258–262. PMID: 16864818.22. Kelley BJ, Rodriguez M. Seizures in patients with multiple sclerosis: epidemiology, pathophysiology and management. CNS Drugs. 2009; 23(10):805–815. PMID: 19739692.23. Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F. ISCVT Investigators. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke. 2008; 39(4):1152–1158. PMID: 18309177.24. Dias L, Soares-Dos-Reis R, Meira J, Ferrão D, Soares PR, Pastor A, et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021; 30(8):105906. PMID: 34111775.25. Fan BE, Shen JY, Lim XR, Tu TM, Chang CC, Khin HS, et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: a black swan event. Am J Hematol. 2021; 96(9):E357–E361. PMID: 34133027.26. Scorza FA, Cysneiros RM, de Albuquerque M, Scattolini M, Arida RM. Sudden unexpected death in epilepsy: an important concern. Clinics (Sao Paulo). 2011; 66(Suppl 1):65–69.27. Stewart M. An explanation for sudden death in epilepsy (SUDEP). J Physiol Sci. 2018; 68(4):307–320. PMID: 29542031.28. Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012; 53(2):227–233. PMID: 22191982.29. Venkatesan A, Johnson RT. Infections and multiple sclerosis. Handb Clin Neurol. 2014; 122:151–171. PMID: 24507517.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Review for Vaccination Guidelines in Patients with Central Nervous System Demyelinating Diseases including COVID-19 Vaccination
- Multiple Abscesses Following COVID-19 Vaccination: A Case Report
- Sudden Cardiac Death Caused by Cardiac Small Vessel Vasculitis after COVID-19 Vaccination (BNT162b2 nCov-19): A Case Report
- The Role of COVID-19 Vaccination for Patients With Atherosclerotic Cardiovascular Disease in the Upcoming Endemic Era
- Psoriasis flares after COVID-19 vaccination: adherence to biologic therapy reduces psoriasis exacerbations: a case-control study