J Korean Foot Ankle Soc.
2023 Jun;27(2):67-70. 10.14193/jkfas.2023.27.2.67.
Multiple Abscesses Following COVID-19 Vaccination: A Case Report
- Affiliations
-
- 1Department of Orthopedic Surgery, Kangdong Sacred Heart Hospital, Seoul, Korea
- KMID: 2543003
- DOI: http://doi.org/10.14193/jkfas.2023.27.2.67
Abstract
- Vaccines can cause adverse reactions, such as soreness, swelling, or redness at the injection site. Some reactions are associated with fever and rash, which are usually mild and transient, and serious side effects are rare. In particular, there are no reports of systemic infection following a COVID-19 vaccination. The authors present a case report of a patient who developed multiple abscesses caused by Staphylococcus aureus after a COVID-19 vaccination. The patient had no previous symptoms or signs of infection. The patient was controlled successfully after surgical and antibiotics treatment.
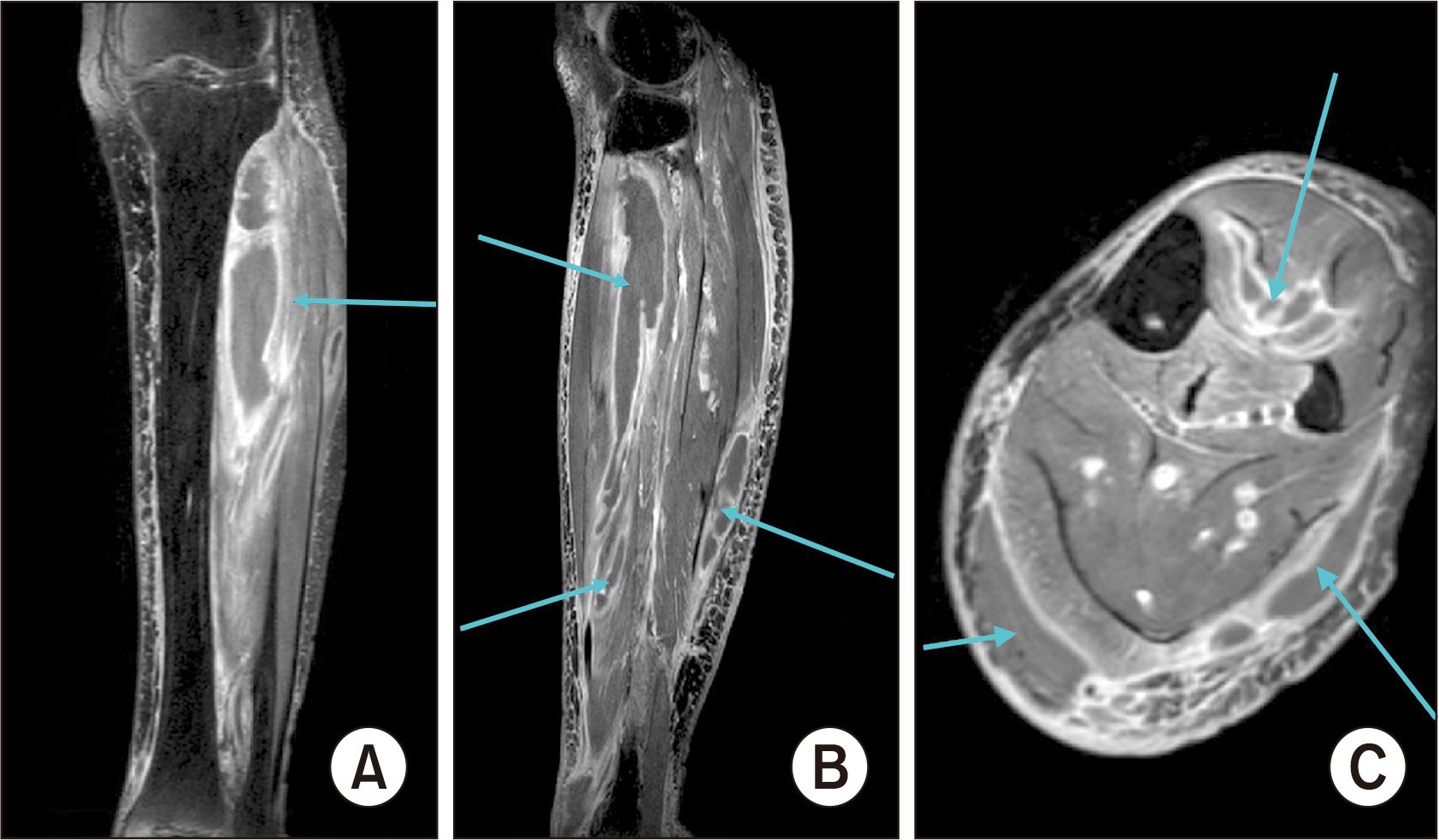
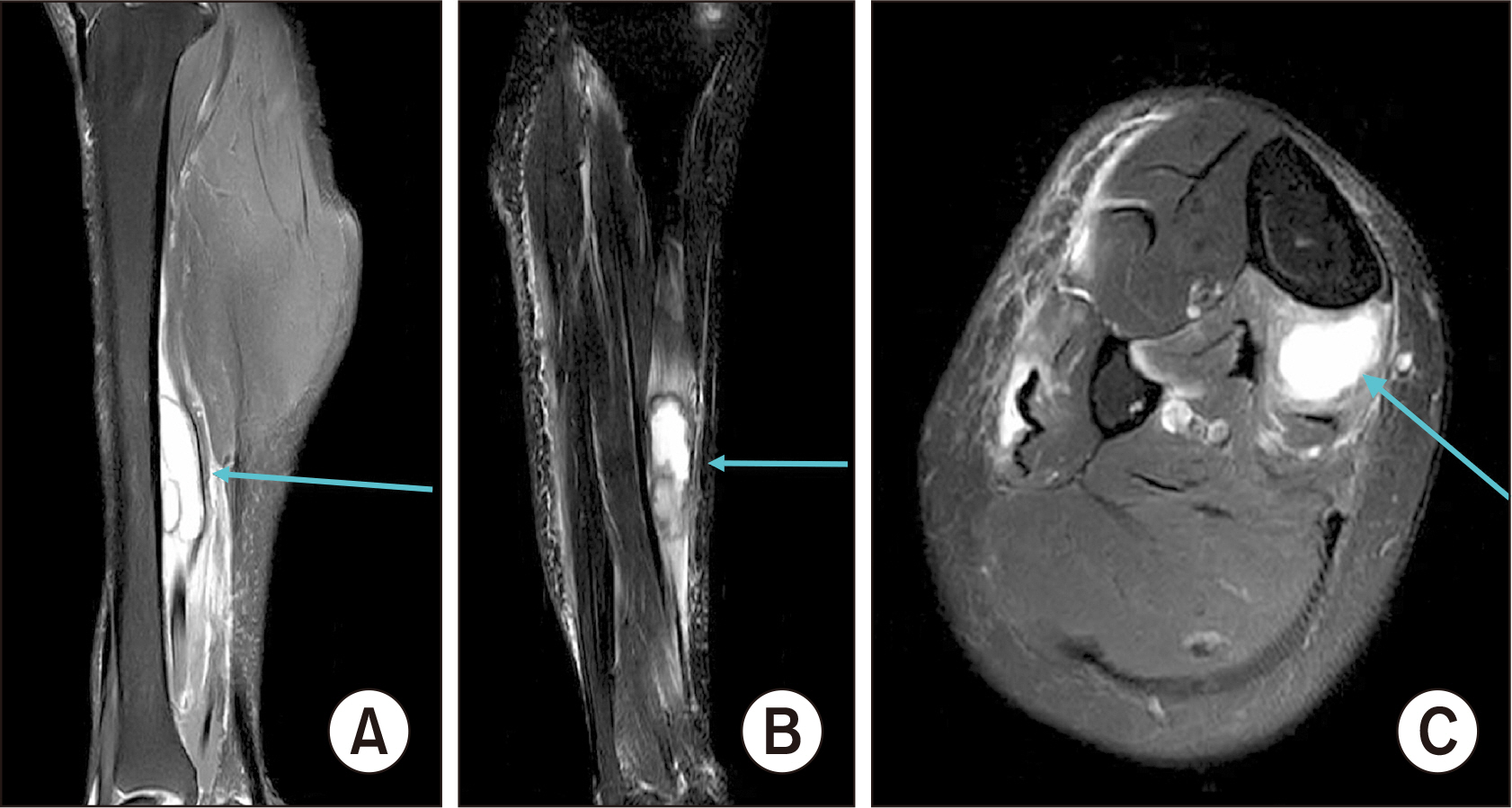
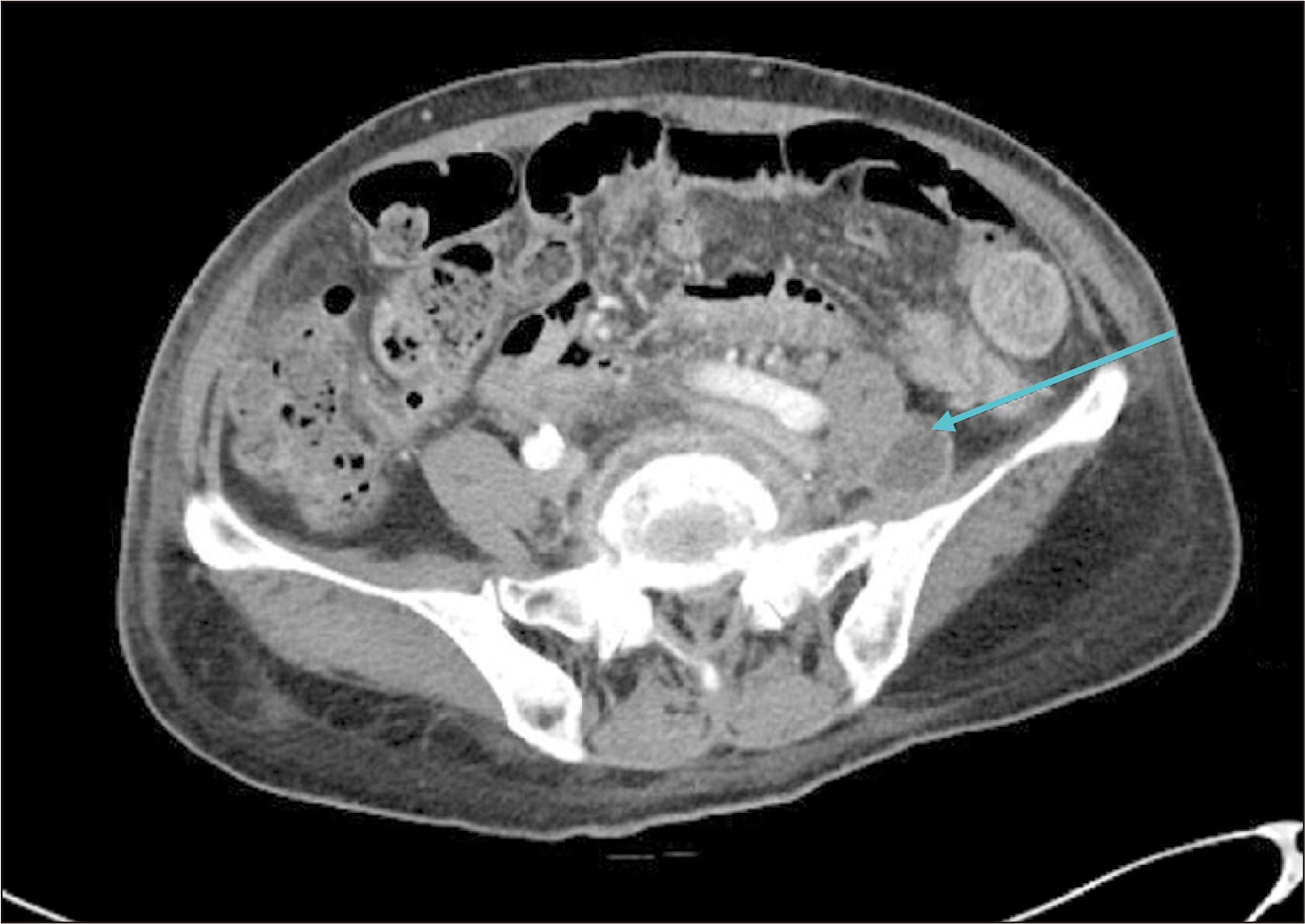
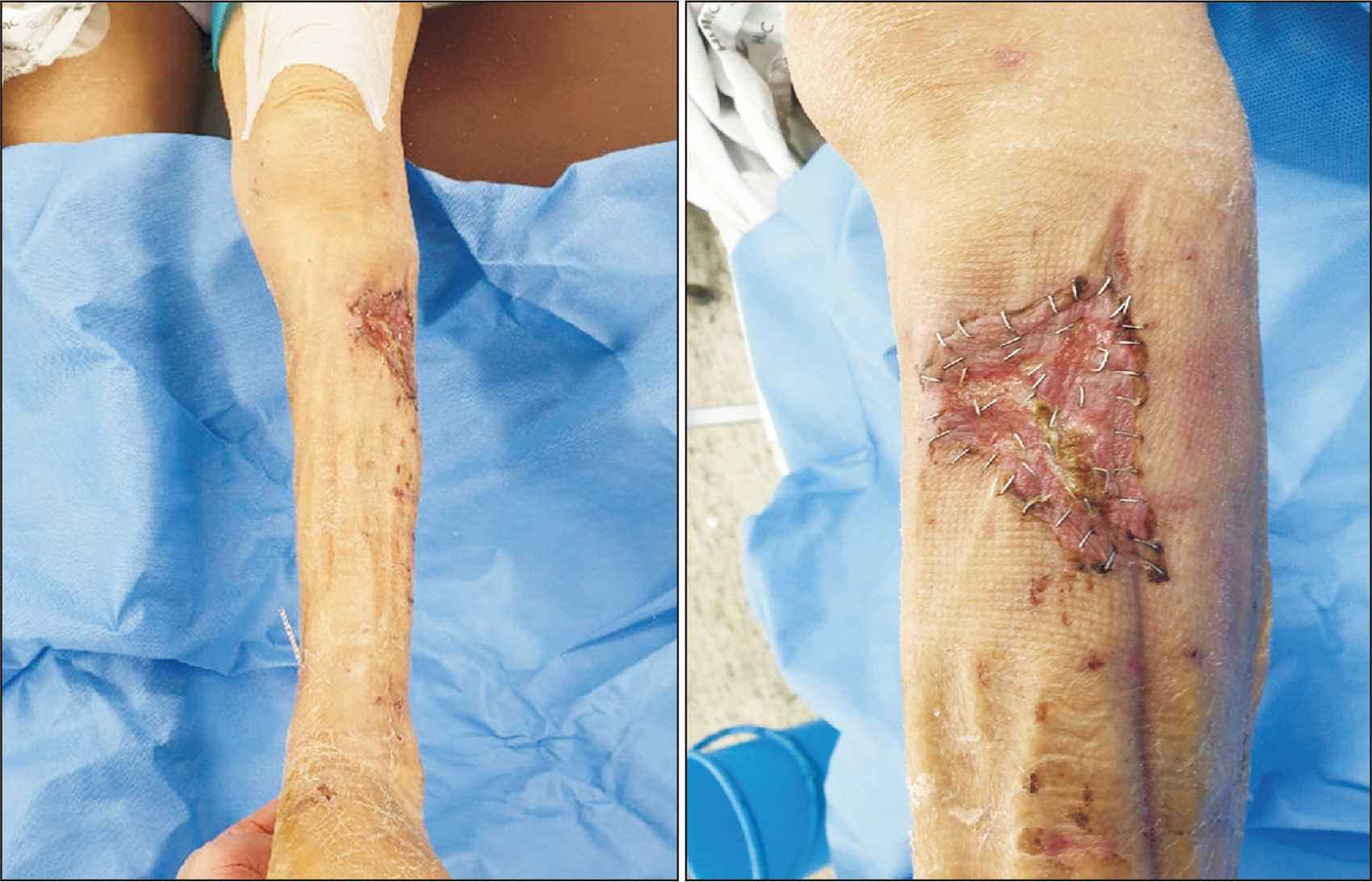
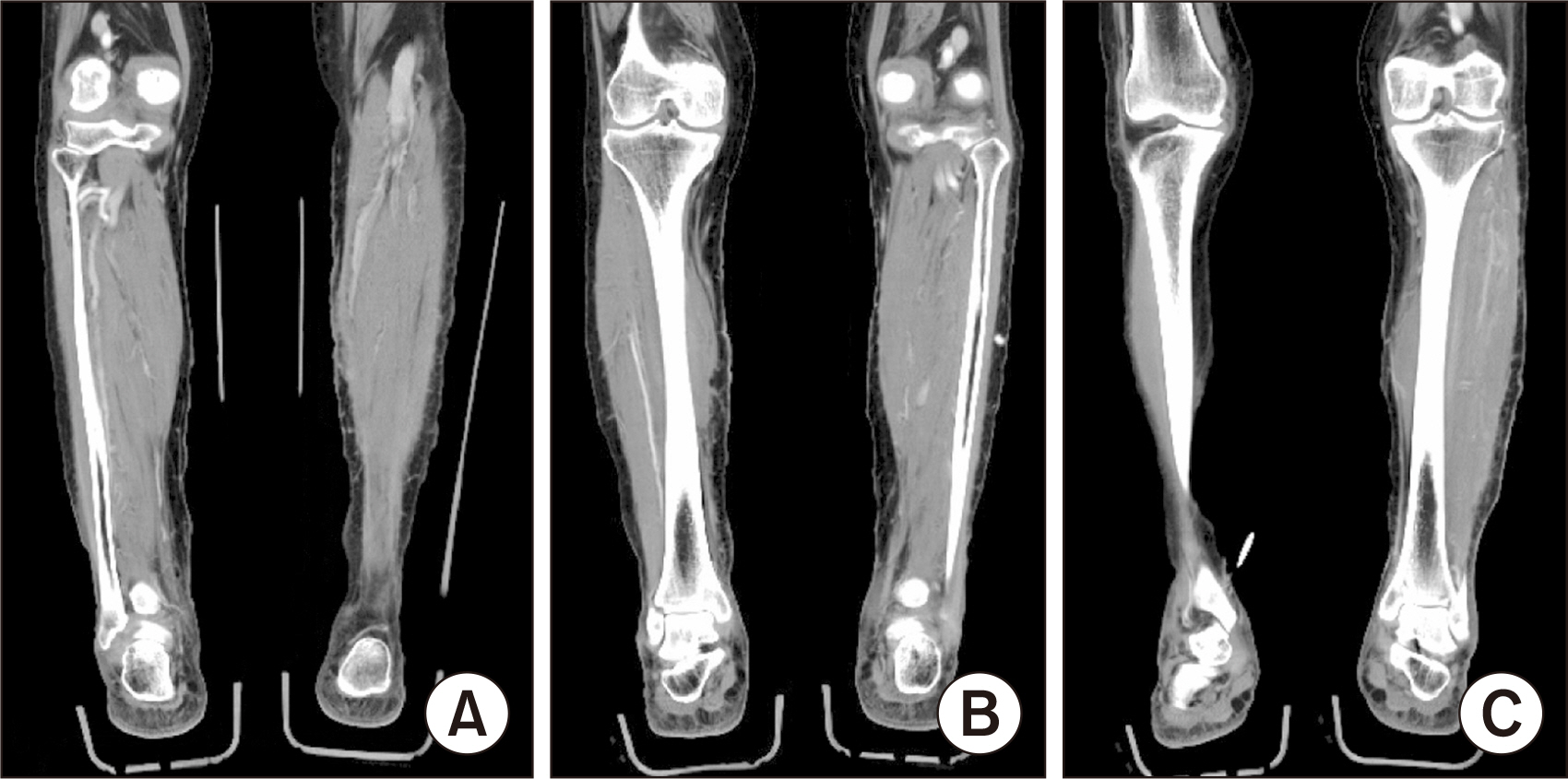
Figure
Reference
-
1. Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. 2021; Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 428:117607. doi: 10.1016/j.jns.2021.117607. DOI: 10.1016/j.jns.2021.117607. PMID: 34365148. PMCID: PMC8330139.
Article2. Cantarelli Rodrigues T, Hidalgo PF, Skaf AY, Serfaty A. 2021; Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA). Skeletal Radiol. 50:2293–7. doi: 10.1007/s00256-021-03803-x. DOI: 10.1007/s00256-021-03803-x. PMID: 33944967. PMCID: PMC8094125.
Article3. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. 2020; Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 383:2603–15. doi: 10.1056/NEJMoa2034577. DOI: 10.1056/NEJMoa2034577. PMID: 33301246. PMCID: PMC7745181.
Article4. Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. 2021; Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 4:e2140364. doi: 10.1001/jamanetworkopen.2021.40364. DOI: 10.1001/jamanetworkopen.2021.40364. PMID: 34935921. PMCID: PMC8696570.
Article5. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. 2021; Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 85:46–55. doi: 10.1016/j.jaad.2021.03.092. DOI: 10.1016/j.jaad.2021.03.092. PMID: 33838206. PMCID: PMC8024548.
Article6. Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. 2022; Innate immune suppression by SARS-CoV-2 mRNA vaccinations: the role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 164:113008. doi: 10.1016/j.fct.2022.113008. DOI: 10.1016/j.fct.2022.113008. PMID: 35436552. PMCID: PMC9012513.
Article7. Rivera-Izquierdo M, Soler-Iborte E, de Rojas JP, Pegalajar-García MD, Gil-Villalba A, Ruiz-Villaverde R, et al. 2021; Factors associated with adverse reactions to BNT162b2 COVID-19 vaccine in a cohort of 3969 hospital workers. Vaccines (Basel). 10:15. doi: 10.3390/vaccines10010015. DOI: 10.3390/vaccines10010015. PMID: 35062676. PMCID: PMC8777949.
Article8. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. 2021; Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 80:1330–8. doi: 10.1136/annrheumdis-2021-220647. Erratum in: Ann Rheum Dis. 2022;81:e133. DOI: 10.1136/annrheumdis-2021-220647. PMID: 34127481. PMCID: PMC8206170.
Article9. Lindgren AL, Austin AH, Welsh KM. 2021; COVID arm: delayed hypersensitivity reactions to SARS-CoV-2 vaccines misdiagnosed as cellulitis. J Prim Care Community Health. 12:21501327211024431. doi: 10.1177/21501327211024431. DOI: 10.1177/21501327211024431. PMID: 34120504. PMCID: PMC8202256.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- A Case of Papulopustular Rosacea-Like Eruptions Following COVID-19 Vaccination
- Early-Onset Myasthenia Gravis Following COVID-19 Vaccination
- Factors Influencing the COVID-19 Vaccination Intentions in Nurses: Korea, February 2021
- A Generalized Lichen Planus Following COVID-19 Vaccination: A Case Report